Abstract
Background
Since there is still no definitive conclusion regarding which non‐steroidal anti‐inflammatory drugs (NSAIDs) are most effective and safe in viral respiratory infections, we decided to evaluate the efficacy and safety of various NSAIDs in viral respiratory infections so that we can reach a conclusion on which NSAID is best choice for coronavirus disease 2019 (COVID‐19).
Methods
A search was performed in Medline (via PubMed), Embase and CENTRAL databases until 23 March 2020. Clinical trials on application of NSAIDs in viral respiratory infections were included.
Results
Six clinical trials were included. No clinical trial has been performed on COVID‐19, Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome infections. Studies show that ibuprofen and naproxen not only have positive effects in controlling cold symptoms, but also do not cause serious side effects in rhinovirus infections. In addition, it was found that clarithromycin, naproxen and oseltamivir combination leads to decrease in mortality rate and duration of hospitalisation in patients with pneumonia caused by influenza.
Conclusion
Although based on existing evidence, NSAIDs have been effective in treating respiratory infections caused by influenza and rhinovirus, since there is no clinical trial on COVID‐19 and case‐reports and clinical experiences are indicative of elongation of treatment duration and exacerbation of the clinical course of patients with COVID‐19, it is recommended to use substitutes such as acetaminophen for controlling fever and inflammation and be cautious about using NSAIDs in management of COVID‐19 patients until there are enough evidence. Naproxen may be a good choice for future clinical trials.
Review criteria
A search was performed in Medline (via PubMed), Embase and CENTRAL databases until 23 March 2020.
Clinical trials on application of non‐steroidal anti‐inflammatory drugs (NSAIDs) in viral respiratory infections were included.
Message for the clinic
Since there is no clinical trial on coronavirus disease 2019 (COVID‐19) and case‐reports and clinical experiences are indicative of elongation of treatment duration and exacerbation of the clinical course of patients with COVID‐19, it is recommended to use substitutes such as acetaminophen for controlling fever and inflammation and be cautious about using NSAIDs in management of COVID‐19 patients until there are enough evidence.
Naproxen may be a good choice for future clinical trials.
1. INTRODUCTION
Currently, coronavirus disease 2019 (COVID‐19) pandemic is the most important challenge the healthcare systems all around the world is facing. Despite the daily increase in the number of COVID‐19 cases 1 and increase in knowledge over COVID‐19, not only no definitive treatment is available for it, but also there is no agreement on its supportive treatment protocol.
Treatments such as antiviral therapy, use of antibiotics, using interferon, corticosteroids and symptomatic treatments such as using anti‐inflammatory and anti‐fever drugs have been mentioned in current guidelines, 2 , 3 some of which have been doubted recently. For example using Lopinavir‐Ritonavir has been mentioned as the first line of antiviral therapy in protocols, but a clinical trial on 199 patients shows that the outcome of using this combination therapy in severe COVID‐19 cases is not different than standard treatment. 4 On the contrary, a case report on four young patients with no underlying illness showed that prescription of ibuprofen exacerbates the condition of the patients. 5 These reports have resulted in studies still seeking the optimum treatment for COVID‐19.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) have been used for management of respiratory infections, controlling fever, reducing chest pain and other symptoms such as cough since a long time ago. 6 , 7 Many NSAID compounds are available, the most famous of which are ibuprofen, diclofenac, aspirin, naproxen and indomethacin. Some of the compounds in this family have anti‐viral properties in addition to anti‐inflammatory properties, which makes them appropriate for treating viral respiratory infections. 8 , 9 Although these medications have been used in respiratory infections for years, there is still no definitive conclusion regarding which compound is most effective and safe.
In a case report on four patients with COVID‐19, it was shown that ibuprofen might exacerbate the condition of patients. 5 However, some guidelines have recommended prescription of ibuprofen in these patients. 2 Therefore, there is still controversy on which NSAID should be used in the treatment protocol of COVID‐19 patients. 10 , 11 , 12 Large number of articles have been published on COVID‐19 in the first 3 months of 2020, most of which have focused on epidemiological evaluation and clinical course and treatment of the disease. In the preliminary search performed by the researchers of the present study it was found that the majority of the studies published on COVID‐19 treatment were case‐series and case‐reports and there are few reports on prescription of NSAIDs in these studies. Therefore, in the present systematic review, we decided to evaluate the effectiveness of various NSAIDs in viral respiratory infections so that we can reach a conclusion on which NSAID is best choice for COVID‐19 based on the findings.
2. METHODS
2.1. Study design
The present systematic review aims to evaluate the existing clinical trials on safety and efficacy of using NSAIDs in management of viral respiratory infections. To reach the aims of the present study, a combination of terms related to NSAIDs and viruses causing respiratory infections were used on search databases. Choosing the keywords related to viruses causing respiratory infections was done with the guidance of a respiratory disease specialist and NSAIDs with the consultation of a pharmacologist.
2.2. Selection criteria
In the present study, all clinical trials were included. Animal studies, in vitro evaluations, non‐viral infections, non‐respiratory infections, observational studies and review studies were excluded.
2.3. Search strategy
A search was performed from the establishment of the database until 23 March 2020. The studied databases included Medline (via PubMed), Embase and CENTRAL. Keywords related to respiratory viruses in combination with NSAIDs were selected based on expert opinion using MeSH and Emtree on Medline and Embase databases and the titles of related articles.
The search strategy used for searching in Medline database has been reported in Appendix A. In addition to the systematic search, a manual search was performed via Google and Google Scholar search engines, and in bibliography of related articles.
2.4. Data gathering
Records were saved in Endnote X7 and duplicates were removed. In the next step, initial screening was performed by two independent researchers. Then, articles were selected based on the inclusion and exclusion criteria, and data were summarised in a checklist by two independent researchers. The gathered data consisted of name of the first author, year of publication, country in which the study was performed, type of study, sample size, age and sex distribution of the patients, name of the virus causing respiratory infection, name of the NSAIDs, dose and duration of prescription, route of administration and treatment outcome. Any disagreement was resolved through discussion with a third reviewer.
2.5. Risk of bias assessment of the studies
Quality control of the studies was performed based on the guidelines of Cochrane Collaboration's tool for assessing risk of bias in randomised trials. 13
3. RESULTS
3.1. Study selection and characteristics
The performed search yielded 1033 non‐duplicate articles. Out of which, six clinical trials were finally included in the present study 14 , 15 , 16 , 17 , 18 , 19 (Figure 1). These studies included 845 adult patients (46.9% male). The studied viruses were rhinovirus in four studies, Influenza type A (H3 N2) in one study and unknown in one study. The NSAIDs applied were naproxen in three studies, aspirin in one study and ibuprofen in two studies. Route of administration was oral in all the studies. The control group was placebo in five studies and oseltamivir in one study. Duration of treatment varied between 4 and 14 days. In three studies, other drugs were used as combination therapy along with NSAIDs. Table 1 depicts the characteristics of the included studies.
Figure 1.
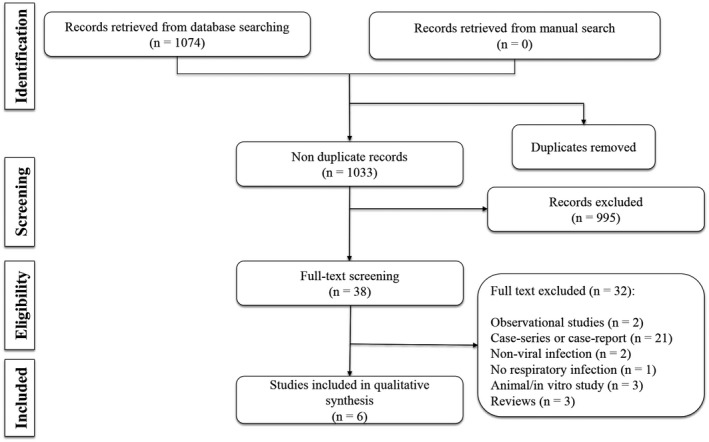
Flow diagram of the present systematic review
Table 1.
Clinical studies that reported the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) in management of viral respiratory infections
| First author; year; country | Study type | Sample size | Age (y) a | Male | Subjects | Treatment groups | Treated patients (n) | Dosage (daily) | Route of administration | Duration of treatment | Combination | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhinovirus | ||||||||||||
| Graham; 1990; Australia 16 | RCT | 60 | 18‐30 | 34 | Rhinovirus infected |
Aspirin, Acetaminophen, Ibuprofen Placebo |
15 15 15 15 |
4 g 4 g 1.2 g |
Oral | 14 d | No | Aspirin and acetaminophen suppressed serum neutralising antibody response and increased patients’ nasal symptoms. Viral shedding was not significantly different. |
| Gwaltney; 1992; USA 18 | RCT | 24 | 21.4 | 14 | Rhinovirus infected |
Naproxen Placebo |
16 8 |
500 mg loading dose, and then, 750 mg for naproxen | Oral | 4 d | INF‐α2b and Ipratropium | Combination of naproxen, INF‐α2b and Ipratropium reduced viral shedding time. Virus titter was lower in treated group. Serum antibody responses and antibody titters were not significantly different. The cold symptoms were reduced in treated subject. |
| Sperbert; 1989; USA 15 | RCT | 49 | 20.5 | NR | Rhinovirus infected |
Ibuprofen Pseudoephedrine Placebo |
18 22 9 |
0.8 g 240 mg NA |
Oral | 4 d | Pseudoephedrine | Illness severity and total symptoms score was reduced in both treated groups compared with placebo. There was no adverse effect directly attributable to ibuprofen. |
| Sperbert; 1992; USA 17 | RCT | 79 | 21.4 | 47 | Rhinovirus infected |
Naproxen Placebo |
39 40 |
400 mg loading dose, and then, 600‐1500 mg for naproxen | Oral | 5 d | No | There was no significant difference in the viral titters and serum antibody responses between the two groups. Symptoms score had significantly improved in naproxen group. |
| Influenza | ||||||||||||
| Hung; 2016; Hong Kong 14 | RCT | 217 |
80 (72‐85) 81.5 (71‐87.3) |
116 | Influenza A (H3 N2) infected with pneumonia |
Naproxen oseltamivir |
107 110 |
1 g daily | Oral | 5 d | Clarithromycin and oseltamivir | Combination treatment of clarithromycin, naproxen and oseltamivir compared with oseltamivir alone significantly reduced 30‐day and 90‐day mortality, and length of hospital stay. |
| Non‐specified | ||||||||||||
| Llor; 2013; Spain 19 | RCT | 416 | 18‐70 | 185 | Non‐pneumonic acute bronchitis |
Ibuprofen Amoxicillin‐clavulanic acid Placebo |
136 137 143 |
1.8 g 1.5 g‐375 mg NA |
Oral | 10 d | No | There is no significant difference in the number of days with cough between groups. Adverse events were more common in antibiotic group than ibuprofen (5%) and placebo (3%). |
Abbreviations: CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IMV, invasive mechanical ventilation; NR, not reported.
Age was reported as range, mean ± SD or median (interquartile range [IQR]).
3.2. Risk of bias assessment of the articles
Risk of bias assessment showed that random sequence generation, blinding of participants and personnel, and other bias had low risk of bias in all studies. Allocation concealment was unclear in two studies. Selective reporting was also unclear in four studies. Finally, blinding of outcome assessment in two studies and incomplete outcome assessment in one study were categorised as high risk (Table 2).
Table 2.
Risk of bias assessment
|
✓ Low risk × High risk ? Unclear |
Graham; 1990 | Gwaltney; 1992 | Hung; 2016 | Llor; 2013 | Sperbert; 1989 | Sperbert; 1992 |
|---|---|---|---|---|---|---|
| Random sequence generation | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Allocation concealment | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Blinding of participants and personnel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blinding of outcome assessment | ✓ | ✓ | × | × | ✓ | ✓ |
| Incomplete outcome assessment | ✓ | × | ✓ | ✓ | ✓ | ✓ |
| Selective reporting | ? | ? | ✓ | ✓ | ? | ? |
| Other bias | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
3.3. The effect of NSAIDs prescription on viral respiratory infection
The included clinical trials had studied different NSAIDs and they had different findings. In the first study, in 1990, Garaham et al performed a clinical trial on 34 healthy volunteers and after infecting them with rhinovirus showed that prescription of aspirin and acetaminophen could inhibit serum neutralising antibody response and increase the respiratory symptoms of these patients. However, these variables were not different from those of the placebo group with ibuprofen prescription. In addition there was no difference in viral shedding between the groups under treatment with acetaminophen, aspirin and ibuprofen combination and placebo. 16 In another study, in 1992, Gwaltney performed a similar study on healthy volunteers and showed that naproxen, interferon‐α2b and ipratropium combination therapy led to decrease in the time of viral shedding and alleviation of the symptoms of cold. In addition, viral titters in this group was lower than the placebo group. However, antibody responses and antibody titters were not different between the two groups. 18 Yet, it is not known that the observed effects are because of naproxen or interferon‐α2b and ipratropium. Anyhow, it seems that naproxen does not cause serious side effects in patients affected with rhinovirus.
In addition, in 1989, Sperber et al studied patients with rhinovirus and showed that oral prescription of ibuprofen decreases the severity of the disease and alleviates cold symptoms in patients compared with the placebo group. Prescription of ibuprofen had no side effects in the studied patients. 15 The same researchers performed another study with similar design in 1992 studying naproxen and showed that 5‐day prescription of naproxen alleviated cold symptoms compared with placebo but had no effect in viral titter and serum antibody responses. 17
3.4. Influenza type A
In their study on 217 Influenza A (H3 N2) patients with pneumonia, Hung et al compared clarithromycin‐naproxen‐oseltamivir combination therapy with oseltamivir alone and expressed that 5‐day therapy with these drugs leads to decrease in 30‐day and 90‐day mortality of patients as well as duration of hospitalisation. 14
3.5. Other viral infection
In 2013, Llor et al studied patients with non‐pneumonic acute bronchitis and expressed that prescription of ibuprofen does not have any effect on the number of daily coughs of patients during 10 days. The rate of adverse effects was reported to be 5% in ibuprofen group and 3% in placebo group. 19
3.6. COVID‐19
In evaluating the effectiveness of NSAIDs prescription in management of COVID‐19, no study was found to point to using these drugs. In the search performed, one clinical trial was found on the effect of prescribing antiviral therapy in management of COVID‐19, which had not mentioned prescription of NSAIDs. 4
3.7. Middle East respiratory syndrome and severe acute respiratory syndrome
No clinical trial was found with the aim of evaluating the effectiveness or safety of NSAIDs prescription in management of Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) patients.
4. DISCUSSION
The present systematic review aimed to evaluate the existing evidence regarding use of NSAIDs in management of viral respiratory. The findings of the present study showed that no clinical trial was performed on patients with COVID‐19, SARS and MERS with this aim. Regarding rhinoviruses, three studies have reported that ibuprofen and naproxen not only have positive effects in controlling cold symptoms, but also do not cause serious side effects. Another clinical trial, which evaluated the effect of clarithromycin, naproxen and oseltamivir combination on pneumonia caused by influenza, showed that this treatment leads to decrease in mortality rate and duration of hospitalisation.
Recently, in a case report, it was revealed that ibuprofen prescription exacerbated the condition of four patients with COVID‐19 and this led the researchers to recommend not consuming it. 5 For further investigation, the researchers of the present study evaluated all the existing reports on treatment of COVID‐19 patients, which led to finding one clinical trial 4 and 12 case‐report/case‐series studies. 1 , 3 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 There was no mention of prescribing NSAIDs in any of these studies. This might be because of no NSAID being prescribed, which is very unlikely; or it might just be that the authors did not point out prescribing NSAIDs. In this regard, it is better that researchers of these studies indicate if they have used NSAIDs in treating the patients or not through a letter to editor or commentary. Further searches revealed that in a study, Jin et al attempted to provide a treatment guideline for COVID‐19 patients. In this study, use of ibuprofen has been suggested for controlling the patients’ fever (fever over 38.5° Celsius). The researchers of the study express that at the time of designing the study, there was not sufficient evidence on COVID‐19. Therefore, the guideline was prepared based on the studies performed on SARS, MERS and Influenza. 2 Based on the search performed for the present systematic review, no clinical trial has been performed on use of NSAIDs in patients with SARS and MERS. Only one study has been performed on influenza type A (H3 N2), which has reported mild to moderate efficacy for naproxen. Therefore, it seems that suggesting consumption of ibuprofen in the mentioned guideline is based on evidence presented in observational studies and not well‐designed clinical trials.
Since the pathophysiology and transmission of COVID‐19 is different from other viruses and it shows significant differences even with other viruses in the same family, such as SARS and MERS, the findings of the studies performed on other viruses may not be generalisable to COVID‐19. In addition, since a case‐report has been recently published that recommended not using ibuprofen in COVID‐19, NSAIDs should be used with caution. Naproxen could be a proper substitute for ibuprofen for controlling patients’ fever. In the clinical trials included in this study naproxen was found to be an effective and safe drug for controlling viral respiratory infections. In addition, there are pre‐clinical evidence that indicate the antiviral properties of naproxen. For instance, Zheng et al showed that naproxen shows wide‐spectrum antiviral effects in mice with influenza. 8 Naproxen inhibits nucleoprotein binding to RNA and consequently inhibits replication in viruses. 35 Since COVID‐19 is an RNA virus, it seems that naproxen is a good option for treating the fever caused by COVID‐19 infection. Therefore, there is a need for designing clinical trials to evaluate the safety and efficacy of naproxen in management of COVID‐19 patients.
5. CONCLUSION
Findings of the present study showed that no clinical trial has been performed with the aim of assessing the efficacy and safety of using NSAIDs in COVID‐19, SARS and MERS infections. In the clinical trials performed on rhinovirus and influenza type A, naproxen has been introduced as a safe and effective treatment for management of respiratory infections. Although based on existing evidence, NSAIDs have been effective in treating respiratory infections caused by influenza and rhinovirus, since there is no clinical trial on COVID‐19 in this regard and case‐reports and clinical experiences are indicative of elongation of treatment duration and exacerbation of the clinical course of patients with COVID‐19, it is recommended to use substitutes such as acetaminophen for controlling fever and inflammation and be cautious about using NSAIDs in management of COVID‐19 patients until there are enough evidence. For performing clinical trials in patients with COVID‐19, naproxen can be a good candidate. Naproxen may be a good choice for future clinical trials.
DISCLOSURE
There is no conflict of interest.
AUTHORS’ CONTRIBUTION
Study design: SS, MY and AZ; Data gathering: MY, AfZ and SS; Interpreting the findings: All authors; Writing the first draft: MY, AMN and SS; Critically revised the manuscript: All authors.
ACKNOWLEDGEMENT
None.
APPENDIX A.
SEARCH QUERIES IN MEDLINE DATABASE
(((randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))) AND (((“Anti‐Inflammatory Agents, Non‐Steroidal”[mh] OR “Cyclooxygenase Inhibitors”[mh] OR “Cyclooxygenase 2 Inhibitors”[mh] OR “Diclofenac “[mh] OR “Ibuprofen “[mh] OR “Indomethacin”[mh] OR “Naproxen”[mh] OR NSAIDs[tiab] OR Non‐Steroidal Anti‐Inflammatory Agents[tiab] OR Diclofenac [tiab] OR Ibuprofen [tiab] OR Indomethacin[tiab] OR Naproxen[tiab])) AND (“Pneumonia”[mh] OR “Pleuropneumonia”[mh] OR “Bronchopneumonia”[mh] OR “Healthcare‐Associated Pneumonia”[mh] OR “Pleuropneumonia”[mh] OR “Pneumonia, Viral”[mh] OR “Respiratory Syncytial Viruses”[mh] OR “Respiratory Syncytial Virus Infections”[mh] OR “Respiratory Syncytial Virus, Human”[mh] OR “Respiratory Syncytial Virus, Bovine”[mh] OR “Rhinovirus”[mh] OR “Influenza, Human”[mh] OR “Influenza A Virus, H10N8 Subtype”[mh] OR “Influenza A Virus, H7N9 Subtype”[mh] OR “Influenza A Virus, H10N7 Subtype”[mh] OR “Influenza A Virus, H7N3 Subtype”[mh] OR “Influenza A Virus, H7N2 Subtype”[mh] OR “Influenza A Virus, H7N1 Subtype”[mh] OR “Influenza A Virus, H1N2 Subtype”[mh] OR “Influenza A Virus, H9N2 Subtype”[mh] OR “Influenza A Virus, H7N7 Subtype”[mh] OR “Influenza A Virus, H5N2 Subtype”[mh] OR “Influenza A Virus, H3N2 Subtype”[mh] OR “Influenza A Virus, H2N2 Subtype”[mh] OR “Influenza A Virus, H1N1 Subtype”[mh] OR “Influenza A Virus, H5N8 Subtype”[mh] OR “Influenza B virus”[mh] OR “Influenzavirus C”[mh] OR “Influenza A virus”[mh] OR “Metapneumovirus”[mh] OR “Parainfluenza Virus 2, Human”[mh] OR “Parainfluenza Virus 3, Bovine”[mh] OR “Bocavirus”[mh] OR “Human bocavirus”[mh] OR “Coronavirus”[mh] OR “Middle East Respiratory Syndrome Coronavirus”[mh] OR “Coronavirus NL63, Human”[mh] OR “Porcine Respiratory Coronavirus”[mh] OR “Coronavirus OC43, Human”[mh] OR “Coronavirus 229E, Human”[mh] OR “Coronavirus Infections”[mh] OR “Coronavirus, Turkey”[mh] OR “Severe Acute Respiratory Syndrome”[mh] OR “SARS Virus”[mh] OR “Alphacoronavirus”[mh] OR “Adenoviridae Infections”[mh] OR “Adenoviridae”[mh] OR “Adenovirus Infections, Human”[mh] OR “Enterovirus”[mh] OR “Enterovirus A, Human”[mh] OR “Enterovirus B, Human”[mh] OR “Echovirus 6, Human”[mh] OR “Echovirus 9”[mh] OR “Enterovirus C, Human”[mh] OR “Enterovirus D, Human”[mh] OR “Herpesvirus 3, Human”[mh] OR “Varicella Zoster Virus Infection”[mh] OR “Hantavirus”[mh] OR “Hantavirus Pulmonary Syndrome”[mh] OR “Hantavirus Infections”[mh] OR “Epstein‐Barr Virus Infections”[mh] OR “Herpesvirus 4, Human”[mh] OR “Parechovirus”[mh] OR “Simplexvirus”[mh] OR “Cytomegalovirus”[mh] OR “Cytomegalovirus Infections”[mh] OR “Measles”[mh] OR “Measles virus”[mh] OR “Morbillivirus”[mh] OR “Morbillivirus Infections”[mh] OR Pneumonitis[tiab] OR Pulmonary Inflammation[tiab] OR Pulmonary Inflammations[tiab] OR Lung Inflammation[tiab] OR Lung Inflammations[tiab] OR Viral Pneumonia[tiab] OR Respiratory Syncytial Virus[tiab] OR Rhinovirus[tiab] OR Coryza Virus[tiab] OR Common Cold Virus[tiab] OR Human Influenzas[tiab] OR Influenza[tiab] OR Influenzas[tiab] OR Human Flu[tiab] OR Flu, Human[tiab] OR Human Influenza[tiab] OR Influenza in Humans[tiab] OR Influenza in Human[tiab] OR Influenza A Virus[tiab] OR H10N8[tiab] OR H7N9 [tiab] OR H10N7 [tiab] OR H7N3 [tiab] OR H7N2 [tiab] OR H7N1 [tiab] OR H1N2 [tiab] OR H9N2 [tiab] OR H7N7 [tiab] OR H5N2 [tiab] OR H3N2 [tiab] OR H2N2 [tiab] OR H1N1[tiab] OR H5N8 [tiab] OR Influenza B virus[tiab] OR Orthomyxovirus Type B[tiab] OR Influenza Virus Type B[tiab] OR Influenza A virus[tiab] OR Influenza Virus Type A[tiab] OR Orthomyxovirus Type A[tiab] OR Orthomyxovirus Type C[tiab] OR Influenza Virus Type C[tiab] OR Influenza C Virus[tiab] OR Influenza C Viruses[tiab] OR avian flu[tiab] OR swine flu[tiab] OR Metapneumovirus[tiab] OR Avian Pneumovirus[tiab] OR Avian Metapneumovirus[tiab] OR Turkey Rhinotracheitis Virus[tiab] OR Human Metapneumoviruses[tiab] OR Human Metapneumovirus[tiab] OR Parainfluenza[tiab] OR Bocavirus[tiab] OR MERS‐CoV[tiab] OR MERS Virus[tiab] OR MERS Viruses[tiab] OR Virus, MERS[tiab] OR MERS[tiab] OR Middle East respiratory syndrome‐related coronavirus[tiab] OR Middle East respiratory syndrome‐related coronavirus[tiab] OR Middle East Respiratory Syndrome Coronavirus[tiab] OR NL63[tiab] OR HCoV‐NL63[tiab] OR Human Coronavirus NL63[tiab] OR HCoV‐OC43[tiab] OR HCoV‐229E[tiab] OR Severe Acute Respiratory Syndrome Virus[tiab] OR SARS‐Related Coronavirus[tiab] OR Coronavirus, SARS‐Related[tiab] OR SARS‐Related Coronavirus[tiab] OR SARS‐CoV[tiab] OR Urbani SARS‐Associated Coronavirus[tiab] OR Coronavirus, Urbani SARS‐Associated[tiab] OR SARS‐Associated Coronavirus, Urbani[tiab] OR Urbani SARS‐Associated Coronavirus[tiab] OR SARS Coronavirus[tiab] OR Coronavirus, SARS[tiab] OR Severe acute respiratory syndrome‐related coronavirus[tiab] OR Severe acute respiratory syndrome‐related coronavirus[tiab] OR SARS‐Associated Coronavirus[tiab] OR Coronavirus, SARS‐Associated[tiab] OR SARS‐Associated Coronavirus[tiab] OR SARS[tiab] OR Alphacoronavirus[tiab] OR Adenoviridae[tiab] OR Adenovirus[tiab] OR Enterovirus[tiab] OR Herpesvirus[tiab] OR Varicella Zoster[tiab] OR Hantavirus[tiab] OR EBV Infections[tiab] OR Epstein‐Barr Virus[tiab] OR Herpesvirus[tiab] OR Parechovirus[tiab] OR Herpes simplex virus[tiab] OR Cytomegalovirus[tiab] OR Measles[tiab] OR Rubeola[tiab] OR Morbillivirus[tiab])).
Yousefifard M, Zali A, Zarghi A, Madani Neishaboori A, Hosseini M, Safari S. Non‐steroidal anti‐inflammatory drugs in management of COVID‐19; A systematic review on current evidence. Int J Clin Pract. 2020;74:e13557. 10.1111/ijcp.13557
Funding information
This study was supported by Shahid Beheshti University of Medical Sciences.
REFERENCES
- 1. Han X, Fan Y, Wan YL, Shi H. A diabetic patient with 2019‐nCoV infection who recovered and was discharged from hospital. J Thorac Imaging. 2020;35:W94‐W95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jin Y‐H, Cai L, Cheng Z‐S, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Day M. Covid‐19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. [DOI] [PubMed] [Google Scholar]
- 6. Schraut J, Keller F, Mertens T, Duprel JB. Influence of the nsaids ibuprofen and diclofenac or paracetamolon kidney function in patients with hantavirus infection. Nephrol Dial Transplant. 2014;29:iii112. [Google Scholar]
- 7. Lewandowska‐Polak A, Brauncajs M, Jarzebska M, et al. Parainfluenza virus infection enhances NSAIDs‐induced inhibition of PGE2 generation and COX‐2 expression in human airway epithelial cells. Adv Med Sci. 2019;64:338‐343. [DOI] [PubMed] [Google Scholar]
- 8. Zheng W, Fan W, Zhang S, et al. Naproxen exhibits broad anti‐influenza virus activity in mice by impeding viral nucleoprotein nuclear export. Cell Rep. 2019;27:1875‐1885.e5. [DOI] [PubMed] [Google Scholar]
- 9. Amici C, Di Caro A, Ciucci A, et al. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir Ther. 2006;11:1021‐1030. [PubMed] [Google Scholar]
- 10. Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID‐19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giollo A, Adami G, Gatti D, Idolazzi L, Rossini M. Coronavirus disease 19 (Covid‐19) and non‐steroidal anti‐inflammatory drugs (NSAID). Ann Rheumatic Dis. 2020:[In press]. [DOI] [PubMed] [Google Scholar]
- 12. Monti S, Montecucco C. ,Non‐steroidal anti‐inflammatory treatment during covid‐19: friend or foe? Response to: 'Coronavirus disease 19 (Covid‐19) and non‐steroidal anti‐inflammatory drugs (NSAID)' by Giollo et al. Ann Rheumatic Dis. 2020:[In press]. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hung IFN, To KKW, Chan JFW, et al. Efficacy of clarithromycin‐naproxen‐oseltamivir combination in the treatment of patients hospitalized for influenza A(H3N2) infection: an open‐label randomized, controlled, phase IIb/III trial. Chest. 2017;151:1069‐1080. [DOI] [PubMed] [Google Scholar]
- 15. Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agent in the treatment of experimental rhinovirus colds. Bull NY Acad Med. 1989;65:145‐160. [PMC free article] [PubMed] [Google Scholar]
- 16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus‐infected volunteers. J Infect Dis. 1990;162:1277‐1282. [DOI] [PubMed] [Google Scholar]
- 17. Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM Jr. Effects of naproxen on experimental rhinovirus colds. A randomized, double‐blind, controlled trial. Ann Intern Med. 1992;117:37‐41. [DOI] [PubMed] [Google Scholar]
- 18. Gwaltney JM Jr, Winther B, Patrie JT, Hendley JO. Combined antiviral‐antimediator treatment for the common cold. J Infect Dis. 2002;186:147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llor C, Moragas A, Bayona C, et al. Effectiveness of anti‐inflammatory treatment versus antibiotic therapy and placebo for patients with non‐complicated acute bronchitis with purulent sputum. The BAAP Study protocol. BMC Pulm Med. 2011;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Q, Quan B, Li X, et al. A report of clinical diagnosis and treatment of 9 cases of coronavirus disease 2019. J Med Virol. 2020;92:683‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei L, Jian‐Ya G. Clinical characteristics of 51 patients discharged from hospital with COVID‐19 in Chongqing, China. medRxiv. 2020: Pre‐Print. 10.1101/2020.02.20.20025536 [DOI] [Google Scholar]
- 25. Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin X, Qiu S, Yuan Y, et al. Characteristics and treatment of patients infected with COVID‐19 in Shishou, China. Lancet Respir Med. 2020:Pre‐Print. Available at SSRN: https://ssrn.com/abstract=3541147. Accessed February 20, 2020. [Google Scholar]
- 27. Shang J, Du R, Lu Q, et al. Treatment and outcomes of patients with COVID‐19 in Hubei, China: a multi‐centered, retrospective, observational study. Lancet. 2020: Pre‐Print. Available at SSRN: https://ssrn.com/abstract=3546060. Accessed March 3, 2020. [Google Scholar]
- 28. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64‐68. [DOI] [PubMed] [Google Scholar]
- 30. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323:1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Li X, Zhang W, Shi Z‐L, Zheng Z, Wang T. Clinical features and treatment of 2019‐nCov pneumonia patients in Wuhan: report of a couple cases. Virol Sin. 2020:[In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Z, Xie S, Zhang J, et al.Short‐term moderate‐dose corticosteroid plus immunoglobulin effectively reverses COVID‐19 patients who have failed low‐dose therapy. Pre‐prints. 2020:Pre‐Print. Available at Preprint: https://www.preprints.org/manuscript/202003.0065/v1
- 35. Dilly S, Fotso Fotso A, Lejal N, et al. From naproxen repurposing to naproxen analogues and their antiviral activity against influenza A virus. J Med Chem. 2018;61:7202‐7217. [DOI] [PubMed] [Google Scholar]


