Abstract
The renin angiotensin system (RAS) plays an important role in the pathogenesis of variety of diseases. Targeting the formation and action of angiotensin II (Ang II), the main RAS peptide, has been the key therapeutic target for last three decades. ACE‐related carboxypeptidase (ACE2), a monocarboxypeptidase that had been discovered 20 years ago, is one of the catalytically most potent enzymes known to degrade Ang II to Ang‐(1‐7), a peptide that is increasingly accepted to have organ‐protective properties that oppose and counterbalance those of Ang II. In addition to its role as a RAS enzyme ACE2 is the main receptor for SARS‐CoV‐2. In this review, we discuss various strategies that have been used to achieve amplification of ACE2 activity including the potential therapeutic potential of soluble recombinant ACE2 protein and novel shorter ACE2 variants.
Keywords: ACE2, ACE2, Angiotensin II, Angiotensin‐(1‐7), Covid‐19, Therapeutic
1. INTRODUCTION
The renin‐angiotensin system (RAS) in its traditional view entails an enzymatic cascade of reactions leading to the generation of angiotensin II (Ang II), the main peptide of the RAS, which has a variety of biological effects. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Angiotensin II is a potent vasoconstrictor and also promotes renal sodium retention, actions that sustain blood pressure and are part of the stress response triggered to maintain circulating volume when survival is threatened by bleeding and other hypovolaemic situations. 7 , 8 , 9 In addition, Ang II has haemodynamic actions that are key to maintain the renal circulation. This peptide, however, exerts a myriad of actions at the tissue level that can be deleterious particularly when sustained chronically. 10 , 11 , 12 Such adverse actions include pro‐inflammatory, pro‐proliferative and pro‐atherosclerotic effects that are independent of its effect on systemic blood pressure and renal haemodynamic actions. 13 , 14 , 15 , 16 , 17 Angiotensin II also increases the production of reactive oxygen species (ROS). This action results from activation of nicotinamide adenine dinucleotide phosphate (NADPH). The increase in ROS contributes to the unwanted effects of this peptide. 18 , 19
RAS blockade based on inhibiting the formation of Ang II with ACE inhibitors or blocking the activation of the Ang II type 1 (AT1) receptor is a widely used therapy for kidney and cardiovascular disease. Pathways that regulate the degradation of Ang II may also be important for determining levels of Ang II, particularly at the tissue level. 20 Until recently, however, little attention had been paid to enhancing Ang II degradation as a way to counteract RAS overactivity which is usually present in kidneys from experimental models of diabetic kidney disease and likely in patients with many causes of CKD. Several enzymes are involved in the degradation of Ang II (Figure 1).
FIGURE 1.
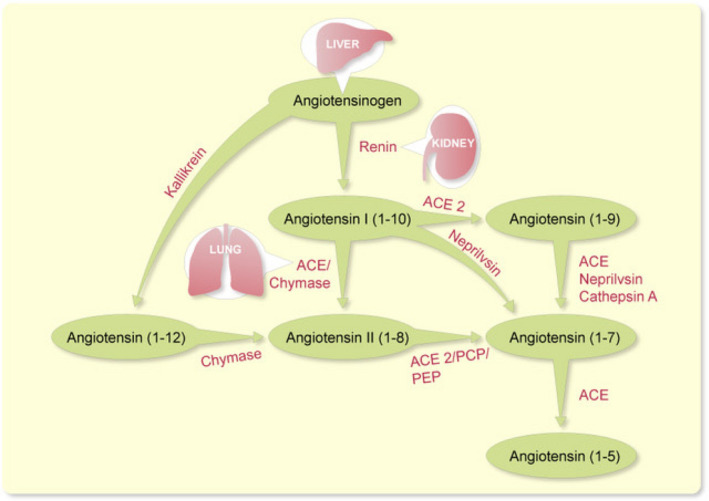
Schematic representation of actions of ACE2 and other enzymes involved in the metabolism of Angiotensin peptides; ACE, angiotensin I–converting enzyme, PCP, prolyl‐carboxypeptidase, PEP, prolyl‐endopeptidase (also known as POP)
In this review we will focus on an ACE‐related carboxypeptidase (ACE2), a homolog of ACE, described in 2000. 21 , 22 ACE2 shares 42% homology with the metalloprotease catalytic domains of ACE. 22 ACE2, unlike ACE, contains only one active domain. 21 , 22 In its full‐length form ACE2 has 805 amino acids, whereas the soluble form of ACE2 has only 740 amino acids. ACE2 is not inhibited by any of the existing ACE inhibitors. ACE2 acts by removing single amino acids from the C terminus of its peptide substrates. 23 , 24 It is one of the more catalytically potent enzymes known to convert the vasoconstrictor Ang II into Ang‐(1‐7) 21 , 23 , 25 Ang 1‐7 is increasingly accepted to have vascular‐protective and reno‐protective properties that oppose and counterbalance those of Ang II, such as vasodilation and oxidative stress. 26 , 27 , 28 , 29 Within the renin angiotensin system, the other known target peptide for ACE2 cleavage is Ang I with the subsequent formation of Ang‐(1‐9). 22 , 23 , 24 , 30 Recent interest in ACE2 has increased dramatically as a result of the recognition that it is the main receptor for SARS‐CoV2, the coronavirus responsible for the current COVID‐19 pandemic.
Studies in experimental models of either genetically or pharmacologically induced ACE2 ablation have generally reported deleterious effects in various organs. Therefore, it is not surprising that over the past several years approaches aimed at augmentation of ACE2 activity had gained a significant interest for their therapeutic potential in a variety of pathological conditions. In this review, we will focus on presenting research done in our laboratory and others using various strategies to achieve amplification of ACE2 activity and discuss its therapeutic implications.
2. ACE2/ANG II/ANG (1‐7) AXIS
Two studies in 2000 reported the existence of a new enzyme that was termed ACE2. 21 , 22 This discovery now 20 years ago created an interest in the ACE2/Ang‐(1‐7) axis. In general, ACE2 and Ang (1‐7) are felt to exert beneficial actions that are organ protective. 21 , 22 , 27 ACE2 and two other peptidases, prolylcarboxipeptidase (PRCP) and prolylendopeptidase (POP), are the known enzymes responsible for the formation of Ang 1‐7 from Ang II (1‐8). 31 , 32 , 33 , 34 These three enzymes cleave the phenylalanine amino acid from the C‐terminal end of Ang II‐(1‐8) to form Ang 1‐7 (Figure 1). The relative importance varies from tissue to tissue. For instance, ACE2 is very critical for this action in the kidney, whereas POP is the dominant enzyme in lungs and systemic circulation. 33 Ang I (1‐10) can also contribute to the formation of Ang (1‐7). The conversion into Ang 1‐7 I from ANG I is produced by neprilysin. 28 , 35 , 36 ACE2 contributes to the formation of Ang (1‐7) not only by enhancing the formation Ang (1‐7) from Ang II (1‐8) but also by increasing the formation of Ang‐(1‐9) from Ang I. Angiotensin (1‐9) could be then converted into Ang 1‐7 by ACE, 22 , 32 neprilysin 37 or cathepsin A. 38 Interestingly, Ang (1‐9) could also contribute to the protective effects since this peptide has been proposed to activate the AT2R 39 (Figure 2).
FIGURE 2.
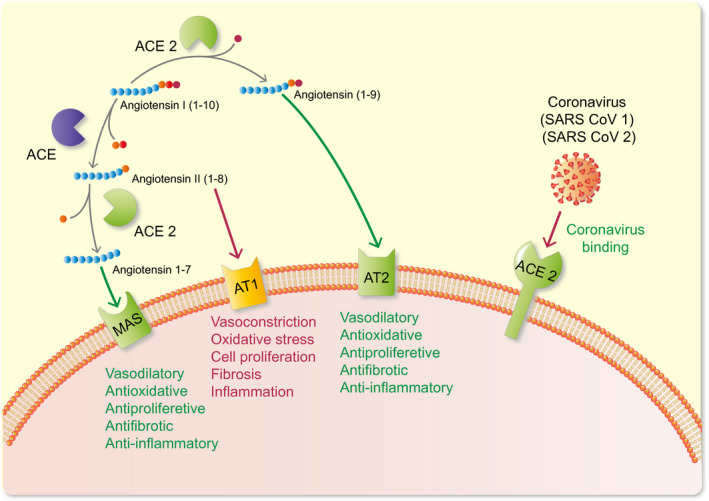
Different receptors in the cell wall and the myriad of actions elicited when activated by the various peptides. ACE2 acts as receptor for the SARS‐CoV‐2 leading to cell viral entry and replication
While this review is limited to the formation of Ang‐(1‐7) from Ang II by ACE2, it should be noted that ACE2 hydrolyzes several other peptide substrates. Those include apelins (ie apelin‐13 and apelin‐36), the opioid peptide dynorphin A, the kallikrein‐kinin‐system peptide des‐ 9 Arg bradykinin and ghrelin, a growth hormone secretagogue. 23 In addition to the catalytic properties of ACE2 this protein may also exert non‐enzymatic functions. 40 Of great interest, the membrane‐bound ACE2 is known to be the receptor for the severe acute respiratory syndrome‐associated coronavirus (SARS‐COV) 41 and recently for SARS‐COV2, the coronavirus responsible for COVID‐19 42 , 43 (Figure 2). Given the rapid emergence of the COVID‐19 pandemic, there is increasing interest in ACE2 and its tissue distribution. This enzyme is only present in small amounts in the circulation but heavily expressed in certain organs such as kidneys and in the intestines. 33 In comparison with these organs, the expression of ACE2 in the lungs is low 33 but present in alveolar type II pneumocytes. 44 Unlike the full‐length ACE2, which is anchored to the plasma membrane, the soluble form of ACE2 circulates but in very small amounts. 25 Theoretically, the administration of soluble ACE2 protein, in sufficient amounts, by binding to the spike protein of COVID‐19, could reduce attachment to the full‐length ACE2 in the plasma membrane. 45 This could be used therapeutically as a way to reduce infectivity in COVID‐19–treated patients.
ACE2 interacts with amino acid transporters 46 and integrins. 47 It is therefore possible that some of the pre‐clinical benefits of ACE2 amplification described below are beyond the dissipation of Ang II and/or the formation of Ang (1‐7). Until more evidence for such other mechanisms become available, however, we are assuming that the conversion of Ang II into Ang (1‐7) is a key step on the mechanism of action. 25 In other words, lowering Ang II and increasing Ang (1‐7) is a therapeutic goal for most biological actions as depicted in Figure 2. Most of the work so far has been done in experimental animals. In healthy human volunteers recombinant ACE2 reduced the level of Ang II. 48 Even though Ang (1‐7) infusion failed to demonstrate vascular effects in humans, 49 Sasaki et al reported that Ang (1‐7) infusion increased forearm blood flow in healthy subjects, whereas in patients with hypertension there was a minimal effect. In human adipose and atrial microvessels, Ang (1‐7) induces vasodilation via nitric oxide (NO)–dependent and telomerase‐dependent processes through MasR, effects that seems to be absent in patients with coronary artery disease. 50
Finally, ACE2/Ang 1‐7/Mas receptor axis undoubtedly seems to have a protective effect on different organ and systems. Interestingly different studies, however, reported that Ang (1‐7) has deleterious effects to increase blood pressure and exacerbate cardiac fibrosis in subtotal nephrectomy rats kidney disease model in association with increased cardiac ACE activity. 51 , 52
3. METHODS OF ACE2 AMPLIFICATION
Several approaches can be used experimentally to increase ACE2 activity (Table 1) those range from ACE2 gene delivery via lentivirus, adenovirus or adeno‐associated virus, creation of transgenic rodent model, minicircle DNA delivery, administration of recombinant ACE2 proteins and the use of ACE2 activators. All of these approaches have been attempted in rodent models and a human recombinant ACE2 protein has been safely administered to humans. Below, we summarize these studies and the pre‐clinical entities that have been studied.
TABLE 1.
Approaches used experimentally to amplify ACE2 activity for therapeutic purposes
|
ACE2 gene delivery via lentivirus, adenovirus or adeno‐associated virus Minicircle DNA delivery Transgenic rodent models Administration of recombinant ACE2 proteins ACE2 activators Novel ACE2 variants of shorter molecular size |
4. VIRAL ACE2 GENE DELIVERY SYSTEMS AND TRANSGENIC MICE
Viral delivery systems using adenovirus, adeno‐associated virus or lentivirus have been used as proof‐of‐concept approaches to augment ACE2 expression in vivo at the central nervous system and variety of peripheral tissues.
4.1. Heart
Huentelman et al 53 first used lentiviral vector encoding mouse ACE2 (lenti‐mACE2) to amplify ACE2 activity in the heart. Lenti‐mACE2 was injected intracardiac in 5‐day‐old Sprague‐Dawley rats. Angiotensin II administration for 4 weeks to control rats resulted in the expected increase in systolic blood pressure, increased weight to body weight ratio and increased myocardial fibrosis. 54 Transduction with lenti‐mACE2 resulted in a decrease in the heart weight to body weight ratio and a reduction in the myocardial fibrosis caused by infusion of angiotensin II. 54 This improvement in cardiac hypertrophy was associated with increased expression of ACE2 in cardiac tissue. Similar to Ang II‐induced hypertension, in the spontaneously hypertensive rat (SHR) murine ACE2 gene transfer into the heart using lentiviral transduction attenuated hypertension and the associated pathological changes, such as left ventricular wall thickness and of perivascular fibrosis. 54 In addition, an improvement of cardiac function, as evidenced by an increase in left ventricular end diastolic and end systolic diameters in SHR rats after ACE2 overexpression, was observed. 54
The use of lentivirus and adeno‐associated viral systems also allowed to generate ACE2‐overexpressing transgenic animals for studies examining effects of cardiac‐specific ACE2 amplification in adult animals. These viral systems, similar to conditional transgenic models, have the advantage of long‐term in vivo ACE2 overexpression that could be “turned on” in adult life and therefore avoid the interference from the effect of developmental ACE2 overexpression. In this respect it should be noted that initial study in traditional transgenic mice with cardiac overexpression of human full‐length ACE2 was not encouraging. 55 Even though transgenic mice appeared healthy, they died prematurely. Their diminished survival rates correlated with the extent of ACE2 overexpression in two transgenic lines suggesting a transgene dose effect. 55 Hearts from both transgenics, however, were essentially normal, without hypertrophy and similar to wild type. In addition, transgenic and non‐transgenic littermates were similar to each other by echocardiography. Moreover, by cardiac catheterization ventricular performance was similar. 55 The mortality of the transgenic mice could ultimately be explained by electrophysiological analyses that revealed conduction disturbances and lethal ventricular arrhythmias in hACE2 transgenic mice. It still remains to be examined whether or not the discrepancy between the cardiac effects by virally‐induced ACE2 overexpression in adult animals and those reported in ACE2 transgenic mice are caused by developmental issues.
4.2. Blood Pressure
Yamazato M et al 56 showed the long‐term effect of ACE2 on blood pressure. In this study, SHR rats had a relative deficiency in ACE2 protein expression within the cardiovascular regulatory neurons of the rostral ventrolateral medulla (RVLM) when compared with normotensive Wistar‐Kyoto rats. Attempted correction of this deficiency in the SHR rats by ACE2 overexpression by lentivirus injection into RVLM resulted in long‐term reduction in blood pressure. 56 Therefore, central ACE2 overexpression could correct its intrinsic decrease in the RVLM and, similarly to the above‐discussed cardiac ACE2 overexpression, 54 leading to a substantial blood pressure reduction in the SHR rat. 56
4.3. Lungs
In lungs, the involvement of RAS in the pathogenesis of certain conditions such as pulmonary hypertension (PH) has been inferred from the high abundance of ACE in the pulmonary vasculature. 57 , 58 High ACE levels likely contribute to excessive generation of Ang II 59 Pulmonary hypertension (PH). Is a disease characterized by a sustained increased in pulmonary artery pressure and resistance over time… 59 Lentiviral overexpression of ACE2 within the lungs was attempted in a mouse model of PH induced by monocrotaline (MCT). In this model, lentiviral vector durably and efficiently transduced ACE2 into a wide variety of cells of lung tissue which was associated with marked attenuation of PH and a reversal of PH‐induced lung injury suggesting support for ACE2 as a possible target for upregulating strategies for the treatment of this disease 59
4.4. Nervous System
It is also important to mention the protective role of ACE2 on the nervous system. Feng et al 60 studied the effects of ACE2 using a transgenic mouse model with high expression of hACE2 protein in the brain. In this study they concluded that overexpression of ACE2 would attenuate the development of neurologic hypertension as a consequence of attenuation of parasympathetic tone and spontaneous baroreflex sensitivity. Of note, neurological deficits improvement and cerebral infarct size reduction after a neurological ischemic event have been attributed to anti‐oxidative and anti‐inflammatory effects of ACE2/Ang (1‐7)/Mas axis. 61 Therefore, ACE2 could also be a target to prevent and treat ischemic stroke in the future.
4.5. Kidneys
Various studies have shown a role of ACE2 in kidney disease. Nadarajah et al generated a model of glomerular ACE2 overexpression using a podocyte‐specific ACE2 transgenic mice and showed partial protection against the early development of albuminuria. 62 Preservation of podocyte proteins and podocyte number was seen in STZ‐induced diabetes transgenic mice with overexpression of the human ACE2 protein. 62 That kidney overexpressed ACE2 can ameliorate glomerular injury in diabetic animals was also suggested by a study using adenoviral kidney ACE2 (Ad‐ACE2) overexpression in STZ rats. 63 Compared with control, the Ad‐ACE2‐treated group showed a reduction in systolic blood pressure and improvement in urinary albumin excretion, creatinine clearance and glomeruli sclerosis index. Ad‐ACE2 also had decreased TGF‐β1, vascular endothelial growth factor and collagen IV protein expression. 63 No additional benefit of ACE inhibition was noticed in the combined use of Ad‐ACE2 and ACEI. 63 Overall these studies suggest that kidney ACE2 amplification may represent a therapeutic target in the treatment of glomerular injury in diabetic kidney disease.
5. MINICIRCLE DNA DELIVERY
Minicircle DNA vectors consist of a circular expression cassette devoid of the bacterial plasmid DNA backbone that provides sustained transgene expression in quiescent cells/tissues. 64 We studied the effects of murine recombinant ACE2 in streptozotocin‐induced diabetes in mice as well as the effect of increasing circulating ACE2 using minicircle DNA delivery. 65 This approach resulted in sustained increase in serum ACE2 activity and enhanced ability to degrade infused angiotensin II (1‐8).. 64 In mice with streptozotocin‐induced diabetes pre‐treated with ACE2, minicircles, plasma ACE2 protein increased as shown by western blot and ACE2 serum activity increased more than 100‐fold. 65 Urinary ACE2 activity and kidney ACE2, however, did not Increase despite the profound augmentation of ACE2 in plasma. Moreover, the glomerular lesions and hyperfiltration seen in this diabetic model of experimental kidney disease were not affected at all. 65 From these findings we concluded that targeting kidney ACE2 rather than circulating plasma ACE2 might be necessary to effectively treat early diabetic kidney disease as seen in the STZ model.
6. RECOMBINANT ACE2
ACE2 in its full‐length form is a 110‐120kDa‐protein comprising 805 amino acids (aa). It is a type I transmembrane protein that contains a major extracellular domain (aa 1‐740), and the much smaller: transmembrane region (aa 741‐768) and intracellular tail (769‐805). 66 , 67 The extracellular domain of ACE2 (1‐740 aa) is enzymatically active as it contains a complete and functional catalytic domain.
7. HUMAN RECOMBINANT ACE2
Thus far, the form of soluble recombinant ACE2 consisting of the extracellular 740 N‐terminal amino acids (aa) has predominantly been used in both pre‐clinical and clinical research. Wysocki et al 25 showed that during Ang II infusion, rACE2 administration effectively degrades Ang II and, in the process, normalizes blood pressure in a mouse model of acute Ang II‐dependent hypertension. (Figure 3). Imai et al 68 used hrACE2 to examine its effect on experimental acute lung injury. Using aspiration‐induced acute lung injury murine model they injected human recombinant ACE2 (hrACE2) into acid‐treated Ace2 knockout mice and observed a decrease in the degree of acute lung injury, and pulmonary oedema formation. hrACE2 has also been proposed as a potential candidate to treat diastolic and systolic heart failure. 69 Zhong et al 70 showed that hrACE2 ameliorated pressure overload induced as well as Ang II‐induced myocardial remodelling. –hrACE2 has also shown to reduce the level of Angiotensin II in healthy human volunteers. Despite the ACE blockade the levels of Angiotensin II could remain elevated via chymase system. 48 All of the above suggest that therapies using hrACE2 for heart failure seem promising.
FIGURE 3.
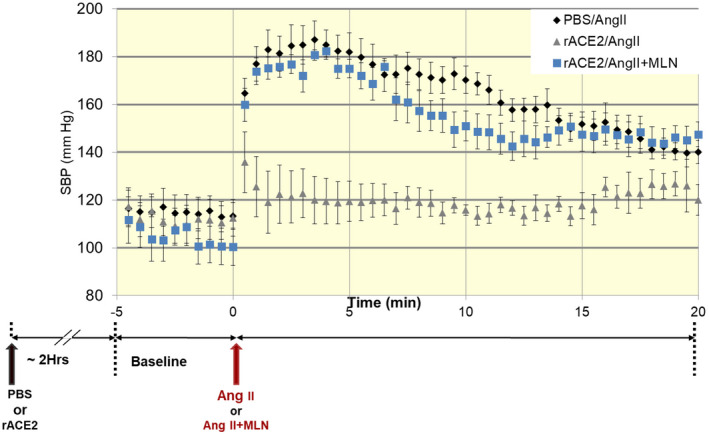
Systolic blood pressure changes after angiotensin II infusion. A bolus of Ang II to mice pre‐treated with PBS (n = 14) was associated with a rapid increase in SBP. In mice treated with rACE2 prior to Ang II injection (n = 11), the SBP increase was blunted and normalized within the first 5 minutes after Ang II injection. Adapted from Wysocki et al Hypertension 2010 25
Human recombinant ACE2 has been evaluated in human subjects in limited clinical studies. The pharmacokinetics, pharmacodynamics, safety and tolerability of hrACE2 were determined in healthy volunteers. 71 This study was randomized, double‐blind and placebo controlled. In addition to showing good tolerability of hrACE2 (1‐740 AA) by healthy human subjects, this study revealed a suppression of systemic Ang II levels both by single and repeated dosing of hrACE2. 71
Since ACE2 has been implicated in animal models of acute lung injury, 68 , 72 it was postulated that administration of hrACE2 could attenuate acute lung injury in human subjects with ARDS. 73 In patients with acute respiratory distress syndrome (ARDS) hrACE2 (GSK2586881) was well tolerated. 73 Human rACE2 caused a decrease of circulating Ang II levels, 73 whereas angiotensin (1‐5) and angiotensin (1‐7) levels were increased and continued elevated for 48 h. 73 In this exploratory study registered under ClinicalTrials.gov, NCT01597635, surfactant protein D (SP‐D) which is considered a beneficial biomarker contributing to normal surfactant structure and inhibition of inflammatory response was increased in hrACE2‐treated subjects compared with placebo. However, hrACE2 infusions did not result in improvement in other physiological or clinical measures of ARDS in this small study and the trial was terminated early. 73
Encouraging but still very preliminary findings were drawn from a recent phase IIa, open‐label pilot study which suggested a potential therapeutic role for hrACE2 in pulmonary arterial hypertension. 74 This study found a reduced plasma ACE2 activity in subjects with PH. This was inferred from higher plasma Ang II to Ang (1‐7) ratio; however, the ratio is not specific as it can potentially be affected by changes in other enzymes that affect the conversion of Ang II into Ang (1‐7). At baseline in subjects with PH, increased expression in six of nine measured cytokines as compared with controls (interleukin (IL)‐10, IL‐1β, tumour necrosis factor (TNF)‐α, IL‐13, IL‐8 and IL‐4) was found. Reduced plasma superoxide dismutase 2 (SOD2), which is considered an anti‐oxidant enzyme, and increased oxidant stress parameters were also observed. 74 After hrACE2, cytokines such as IL‐10, IL‐1β, IL‐2 and TNF‐α were decreased 2 hours after administration. Human rACE2 administration was reported to also beneficially influence SOD2 levels, and reduce plasma oxidant stress. These findings were based on a limited number of subjects. Further assessment of hrACE2 as a potential therapeutic in PH certainly will require larger studies. Table 2 provides a summary of studies so far that have used human soluble recombinant ACE2.
TABLE 2.
Studies in human subjects receiving recombinant soluble ACE2
| Title | NCT number | Number of participants | Condition or disease | Intervention/Treatment | Collaborators/Sponsors | Phase | Status |
|---|---|---|---|---|---|---|---|
| A two part study to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2586881 in patients with acute lung injury | NCT01597635 | 44 participants | Lung injury, Acute |
Drug: Dose 4 GSK2586881 Drug: Placebo (saline) |
GlaxoSmithKline | Phase 2 | Completed |
| An open‐label, dose‐escalation study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of single Doses of GSK2586881 in participants with pulmonary arterial hypertension | NCT03177603 | 23 participants | Hypertension, pulmonary | Drug: GSK2586881 | GlaxoSmiithKline | Phase 2 | Completed |
| The effects of GSK2586881 on the responses to acute hypoxia and exercise | NCT03000686 | 17 participants | Healthy volunteers |
Biological:GSK2586881 Other: Placebo |
GlaxoSmithLline | Phase 1 | Terminated |
| A Randomized, open label, controlled clinical study to evaluate the recombinant human angiotensin‐converting enzyme 2 (rhACE2) in adult patients with COVID‐19 | NCT04287686 | 0 participants | COVID‐19 | Drug: Recombinant human angiotensin‐converting enzyme 2 (rhACE2) | The first affiliated hospital of Guangzhou Medical University | Not applicable | Withdrawn (without CDE approval) |
8. RODENT RECOMBINANT ACE2
While the human rACE2 is the ultimate form of the protein that can be used in clinical studies, it has some limitations for pre‐clinical research in rodents. Human rACE2 can certainly be used in acute studies in rodents. There is a limited value, however, for chronic studies as a result of the development of antibodies that decrease the enzymatic activity of ACE2 after 2 weeks of administration to mouse models. 25 , 75 Mouse rACE2 should not elicit formation of such neutralizing antibodies when given to mice, Indeed, we documented sustained elevations in serum ACE2 activity after murine rACE2 administration, 75 thereby providing a suitable strategy for ACE2 amplification in chronic studies in mice. One has to keep in mind, however, that mouse and human rACE2 are not identical in terms of their enzymatic effects on RAS peptides. While both mouse and human recombinant ACE2 promote the degradation of Ang II and lead formation of Ang (1‐7), 75 , 76 only human recombinant ACE2 degrades Ang I to form Ang‐(1‐9). 75 , 76
Experimentally, mouse intact (1‐740) rACE2 had been employed to assess its effects on hypertension induced in mice by Ang II. In this respect, single injection of soluble rACE2 caused a marked increase in circulating ACE2 activity and, after supra physiological dose of Ang II (1ug/g body weight), within few minutes circulating levels of this peptide normalized and blood pressure fell more rapidly than in animals not infused with ACE2. 75 Intact (1‐740) of Human Recombinant ACE2 has also been used for similar experiments showing similar results. 25 Of note, the infusion of rodent recombinant ACE2 under baseline conditions does not lower blood pressure. 75
That the effects of rACE2 are restricted to the circulation was suggested by the observations that its administration did not result in noticeable increase in ACE2 activity within the heart, 25 , 65 the kidney 25 , 65 or in the urine 25 , 65 , 77 of healthy mice. In diabetic mice with only modest albuminuria rACE2 also did not appear in the urine. 65 , 77 In contrast, in the Alport mouse model of severely compromised glomerular filtration barrier, we found a massive increase in urinary ACE2 after soluble rACE2 administration. 65 The large molecular size of soluble rACE2 (100‐110 kDa) 65 , 77 renders it not filterable trough an intact or only slightly damaged glomerular barrier. In addition, soluble rACE2 1‐740 tends to form homodimers making its actual size even bigger and thus reduce further the likelihood of glomerular basement membrane passage. 76 , 77
rACE2 administration to markedly increase circulating levels of soluble ACE2 can effectively obliterate Ang II‐induced hypertension 25 , 75 , 78 or hypertension caused by renin overexpression mouse models. 79 Therefore, it is conceivable that the use of soluble rACE2 could likely be applied to treatment of conditions associated with systemic RAS activation that secondarily might lead to kidney injury. In contrast, in states where local kidney RAS is expected to be overactive, such as diabetic kidney disease, 80 soluble rACE2 of 1‐740 AA might not be effective. Consistent with this notion, we have found that in STZ‐treated mice, a diabetic mouse model with local kidney but not systemic RAS overactivity, 65 long‐term augmentation of circulating ACE2 activity was not sufficient to beneficially alter albuminuria, GFR or kidney histology. 65 Glomerular filtration of administered rACE2 is a prerequisite for a subsequent tubular uptake of the protein from the urinary space. 65 , 80 Kidney targeting of biologics is complex and can be affected by many factors including their molecular size. 81 We reasoned that in forms of kidney disease with an overactive RAS within the kidney, 65 , 82 ACE2 amplification would require forms of recombinant ACE2 that are short enough to be able to pass the glomerular filtration barrier and be consequently reabsorbable by the kidney to be able to exert any direct kidney‐specific therapeutic effect. Based on these considerations, we have generated ACE2 variants of shorter molecular size that still retain enzymatic activity (see below).
9. NOVEL VARIANTS OF ACE2 OF SHORTER MOLECULAR SIZE
We have generated and tested two novel recombinant mouse ACE2 proteins of a molecular size (~69‐71 kD) which are much shorter than the original soluble rACE2 of 100‐110 kD. The two short ACE2 variants 1‐605 and 1‐619 AA have been found to be active systemically and of a molecular size short enough to render them filterable through the kidney glomerular filtration barrier. 65 , 80 Moreover, we have found that these short variants could by the kidney tubules and thus capable to amplify kidney ACE2 activity and foster the formation of Ang (1‐7) from Ang II. This feature should make them attractive to combat RAS activation in a vast array of kidney diseases where the RAS is overactive, such as the acute kidney injury.
The pharmacokinetics of two active short ACE2 proteins 1‐619 and 1‐605 was examined in mice after iv and ip administration based on measurements of serum ACE2 activity against a fluorogenic substrate Mca‐APK‐Dnp. 65 , 80 After intravenous administration, ACE2 1‐619 and 1‐605 showed a substantially extended circulatory elimination half‐life (~4 hours) as compared to the elimination half‐life of the original soluble rACE2 (~ 1.4 hours). After ip administration, the elimination half‐life of the original soluble rACE2 was similar or slightly longer than that of ACE2 1‐619 and 1‐605 variants. The area under the curve and the peak plasma ACE2 activity for both short ACE2 proteins after iv injection and after ip administration, however, were considerably higher than that of the original soluble rACE2 suggesting overall improved pharmacokinetic profiles as compared to the original soluble rACE2. Even though the pharmacokinetic data show clear superiority of short rACE2 variants as compared to the original soluble rACE2, it should be cautioned that this effect may be dependent on the artificial substrate used to measure ACE2 activity. 65 , 80
Catalytic efficiency towards the fluorogenic substrate Mca‐APK‐(Dnp) was also found to be much higher for both short rACE2 variants than that of the original soluble rACE2 which might, in part, reflect higher affinity towards this artificial substrate. 65 , 80 Against the natural substrate, Ang II, the catalytic efficiency was similar for the short ACE2 variants and the original soluble ACE2. Likewise, the two short ACE2 variants 1‐605 and 1‐619 in vivo exhibited a similar systemic effect on the acute Ang II‐induced hypertension as the original soluble ACE2. 65 , 80 Both small ACE2 variants blunted the peak increase in blood pressure after Ang II infusion which normalized within 5 minutes or less to a similar extent as the original soluble ACE2. 65 , 80 Therefore, both small ACE2 variants seem to degrade excess of systemic circulating Ang II to a similar degree as original soluble rACE2, thereby enhancing blood pressure recovery when Ang II is infused.
In addition to the systemic effect, both small ACE2 variants have also an added effect on the local urinary and kidney ACE2 activity augmentation which is in contrast to the original soluble ACE2. 65 , 80 This was recently demonstrated in experiments where short ACE2 variants and the original soluble ACE2 were administered to ACE2‐deficient mice. In contrast to original soluble rACE2, both small recombinant variants resulted in a gain in urinary ACE2 activity. L‐lysine, a tubular reabsorption blocker, 83 , 84 further increased urinary ACE2 activity suggesting that the two short ACE2 variants are both filtered and reabsorbed by the tubules. Moreover, it was demonstrated that the short ACE2 protein variant 1‐619 is taken up by the kidney in enzymatically active state. This was shown by the presence of ACE2 activity in kidneys isolated from ACE2‐deficient mice that had been previously injected with ACE2 1‐619 but not from the kidneys injected with equivalent dose of original soluble rACE2. In addition, ex vivo kidney cortex lysates from ACE2 1‐619‐injected mice were able to form significantly more Ang (1‐7) from Ang II than kidney lysates from PBS‐ or original soluble rACE2‐injected mice. In the aggregate these data demonstrate that short ACE2 variants are active, and sufficiently small to be filtered by the kidney and moreover capable to increase kidney ACE2 activity to an extent that Ang (1‐7) formation from Ang II is increased.
10. ACE2 ACTIVATORS
The crystal structure of ACE2 was solved by Hernández Prada et al 85 and three putative small molecule binding pockets were identified. These authors carried out in silico screening of a small molecule library followed by in vitro studies and found that of the three sites, two of them were inhibitor sites and the third one was a presumed activator site. The same investigators 85 identified then two small‐molecular compounds as ACE2 activators: XNT and resorcinolnaphthalein. XNT was preferred over resorcinolnaphthalein since XNT solubility properties appeared to be more favourable. 85 Three years later an agent commonly used to treat some parasitizes in animals 86 called Diminazene (DIZE) was proposed as an ACE2 activator. 87 Both XNT and DIZE have been used experimentally to serve as potential treatment for various conditions such as certain types of hypertension, 85 , 86 , 87 , 88 pulmonary hypertension, 89 , 90 , 91 Cardiac and renal fibrosis 85 and glaucoma. 92 The therapeutic benefits of these two components should be attributable to conversion of Ang II into Ang (1‐7) as a consequence of ACE2 activation. 86 , 87 , 89 , 90 , 91 , 92 One of the caveats with many of these studies is, however, that the effect on ACE2 activity was usually not reported in vivo. 85 , 87 , 89 , 90 , 91 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 In these studies, moreover, it was not shown that the use of these presumed ACE2 activators had taken place by demonstrating the enhanced conversion of Ang II into Ang (1‐7).
Our group reported low levels plasma ACE2 activity in both vehicle and XNT‐infused mice. 65 , 80 Furthermore, after Ang II infusion, the plasma levels Ang (1‐7), the peptide generated by the cleavage of Ang II and plasma levels Ang II, the substrate of ACE2, were not affected by the administration of XNT. Therefore, it was suggested that the effect of XNT on Ang II‐induced hypertension was not caused by activation of ACE2. To substantiate this hypothesis, we performed experiments in an ACE2 KO mouse model. Despite the lack of ACE2, XNT was capable to elicit an enhanced recovery from hypertension induced by Ang II infusion similar to the recovery seen in WT mice (45). In addition, XNT and DIZE failed to increase ACE2 enzymatic activity of either mouse or human rACE2 in vitro and ex vivo using mouse and rat kidney tissue. Consistent with these findings, Ang II degradation ex vivo and in vitro was not affected by XNT and DIZE. 78 It should be also noted, however, that XNT and DIZE have been reported to cause a significant increase in ACE2 mRNA expression. 99 , 101 , 102 This suggests that these two compounds could upregulate ACE2 gene expression, leading to an ACE2 activity augmentation chronically. Although the acute (minutes) effect of XNT on blood pressure that Haber et al 78 reported in ACE2 replete mice after Ang II infusion seems unlikely to be explained by this upregulation of ACE2 mRNA expression. Recently, an egg white‐derived tripeptide IRW (Ile‐Arg‐Trp) was reported to increase ACE2 activity of hrACE2 in a cell‐free system and also to upregulate ace2 mRNA expression in cultured cells. 103 There have been no follow‐up studies, to our knowledge, with the IRW compound which would independently assess its potency as an ACE2 activator. As of today there is no convincing evidence, in our opinion, that the presumed ACE2 activators studied exert their otherwise undisputable biological effects by activating ace2 as their main mechanism of action.
11. POTENTIAL THERAPEUTIC ACTION OF ACE2 AMPLIFICATION
From the forgoing, it is logical to postulate that ACE2 amplification within the kidney is a very attractive therapeutic approach to promote the metabolism of Ang II and reduce its detrimental actions. Some of the potential diseases that could benefit from ACE2 amplification have been mentioned already while discussing the methods for increasing ACE2 activity. As noted earlier, partial kidney protection against STZ‐induced diabetic kidney disease could be shown in a transgenic model where ACE2 overexpression was limited to glomerular podocytes. 62 In one study human recombinant ACE2 was shown to improve diabetic kidney disease in Akita mice. 104 When these mice were injected with hrACE2 for 4 weeks, hrACE2 normalized blood pressure and reduced albuminuria. Unlike other models of early DKD, the Akita mouse is hypertensive and is very possible that the improvement in albuminuria noted in this study was related to lowering of blood pressure by ACE2 by decreasing Ang II which is elevated in this model.
In keeping with previous studies in mice by Ye et al, 105 Mizuiri et al 106 found that in patients with diabetic kidney disease expression of ACE and ACE2 was altered. In this study, ACE2 expression was decreased while levels of ACE expression were increased in glomeruli, resulting in a significant increase in the ACE/ACE2 ratio. Reich et al 107 measured ACE2 and ACE mRNA expression in kidney biopsies from patients with type 2 diabetes and associated kidney disease and controls. Glomerular and proximal tubular ACE2 mRNA expression was reduced by more than half while ACE mRNA was augmented in both compartments in diabetic patients compared to controls. This finding suggests that ACE2 gene expression may play an important role in the development and progression of kidney injury in human subjects with diabetes.
Wysocki et al 65 examined the kidney effects of mrACE2 in mice with STZ‐induced diabetic nephropathy as well as the amplification of circulating ACE2 using minicircle DNA delivery prior to induction experimental diabetes. Recombinant murine ACE2 given for 4 weeks failed to stop the progression kidney pathology and albuminuria in this model. A markedly increase in ACE2 activity as well as an enhanced ability to metabolize acute load of Ang II resulted from ACE2 minicircle delivery. However, this augmented ACE2 activity achieved by this technique did not affect urine ACE2 activity. Moreover, there were no improvements in albuminuria, glomerular expansion, glomerular cellularity or glomerular size when compared to vehicle‐treated diabetic controls (Figure 4). Therefore, this study emphasized the importance of targeting the kidney rather the circulating RAS in order to treat diabetic nephropathy.
FIGURE 4.
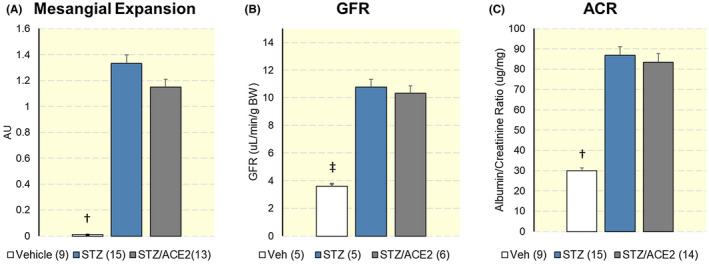
A, Mesangial expansion score, B, Glomerular filtration rate (GFR, middle) and C, albumin/creatinine ratio (ACR, right) in mice 20 weeks after diabetes induction with streptozotocin (STZ) and in vehicle‐treated (Veh) mice that served as a non‐diabetic control group. There were no differences in any of the parameters examined. Between ACE2‐treated and untreated animals. †P < .01 ‡P < .001. Adapted from Wysocki et al, Kidney International, 2017 65
In other study using Col4a3 ‐/‐ mouse model of Alport's syndrome, murine recombinant (mr) ACE2 also decreased markers of kidney injury. ACE2 plasma activity was increased using mrACE2 minipumps leading to an improvement in albuminuria, reduced ERK1/ERK2 signalling and amelioration of inflammation, fibrosis and oxidative stress. 108 In this study renal function did not improve as measured by blood urea nitrogen and GFR was not measured. In this model, the profound defect in glomerular permeability allows the infused rACE2, despite its large molecular size, to get filtered. 65 We think that part of the benefit observed may have reflected local kidney uptake of the infused ACE2. Clearly, in a model of early DKD systemic administration of ACE2 did not prevent the development of hyperfiltration or mild diabetic kidney injury as observed in STZ‐treated animals. 65 As pointed out by us 65 and others 109 in order to achieve an effective therapeutic approach, increasing circulating ACE2 levels may not be enough and other strategies that modulate the ACE2/Ang (1‐7)/MasR axis using molecules capable to reach the glomerulus should be considered. We are encouraged by the development of novel ACE2 variants of shorter molecular size that can be filtered by the kidney and able to increase the formation of Ang (1‐7) from Ang II. 80 The therapeutic potential of these short ACE2 variants is currently being explored in pre‐clinical studies for acute and chronic kidney injury. Alterations in the Renin Angiotensin system (RAS) are involved not only in the progression of chronic kidney disease (CKD) 110 , 111 , 112 , 113 , 114 but also in AKI and are associated with adverse outcomes both in experimental and clinical studies. 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 We think that AKI could be an initial target to explore in humans the potential preventative effect of our short ACE2 variants.
Finally, this review comes at a time that the world is grappling with the COVID‐19 pandemic caused by SARS‐CoV2 virus. From the SARS outbreak in 2003 we have known that SARS‐CoV1 spike glycoprotein recognized ACE2 as a receptor on the cell surface for host entry. 41 Consequently, there have already been several reports looking at the association of SARS‐CoV2 and ACE2. 43 , 123 It is known that SARS‐COV2 does bind to ACE2 to gain host cell entry causing its cell internalization and likely reducing membrane ACE2 expression. 124 In murine models, the dysregulation of ACE2 is associated with cardiac, pulmonary and kidney alterations. There is preliminary data from COVID‐19 subjects in whom elevated levels of plasma angiotensin II correlated with degree of lung injury. 125 Further prior pre‐clinical studies in respiratory syncytial virus and avian H5N1 influenza suggested that restoration of ACE2 by recombinant ACE2 administration appeared to reverse worsening lung injury. 73 , 126 , 127 Therefore, there is now significant interest in looking at recombinant ACE2 protein to rebalance the RAS network and potentially help mitigate the pulmonary, cardiac and kidney damage done by COVID‐19. We have proposed that soluble ACE2 may act as a competitive interceptor of SARS‐CoV and SARS‐CoV2 by preventing binding of the viral particle to the surface‐bound, full‐length ACE2 45 . In this context, administration of soluble recombinant human ACE2 proteins might be beneficial as novel biologics to treat the infection caused by coronaviruses that utilize ACE2 as a receptor.
CONFLICT OF INTEREST
D. Batlle and J. Wysocki: co‐inventors Patent: ‘Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2’; and have also submitted a patent on the potential use of novel ACE2 proteins for coronavirus infection. D. Batlle: Founder of ‘Angiotensin Therapeutics’.
DISCLOSURE
The other authors have nothing to disclose relevant to this manuscript.
Marquez A, Wysocki J, Pandit J, Batlle D. An update on ACE2 amplification and its therapeutic potential. Acta Physiol. 2020;231:e13513. 10.1111/apha.13513
REFERENCES
- 1. Ito M, Oliverio MI, Mannon PJ, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92(8):3521‐3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacoby DS, Rader DJ. Renin‐angiotensin system and atherothrombotic disease: from genes to treatment. Arch Intern Med. 2003;163(10):1155‐1164. [DOI] [PubMed] [Google Scholar]
- 3. Ruiz‐Ortega M, Lorenzo O, Rupérez M, et al. Role of the renin‐angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38(6):1382‐1387. [DOI] [PubMed] [Google Scholar]
- 4. Almeida‐Santos AF, Kangussu LM, Campagnole‐Santos MJ. The renin‐angiotensin system and the neurodegenerative diseases: A brief review. Protein Pept Lett. 2017;24(9):841‐853. [DOI] [PubMed] [Google Scholar]
- 5. Dostal DE, Baker KM. The cardiac renin‐angiotensin system: Conceptual, or a regulator of cardiac function? Circ Res. 1999;85(7):643‐650. [DOI] [PubMed] [Google Scholar]
- 6. Griendling KK, Ushio‐Fukai M, Lassègue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. Hypertension. 1997;29(1):366‐370. [DOI] [PubMed] [Google Scholar]
- 7. Anderson S, Brenner BM. Therapeutic implications of converting‐enzyme inhibitors in renal disease. Am J Kidney Dis. 1987;10(1 Suppl 1):81‐87. [PubMed] [Google Scholar]
- 8. Ichikawa I, Ferrone RA, Duchin KL, Manning M, Dzau VJ, Brenner BM. Relative contribution of vasopressin and angiotensin II to the altered renal microcirculatory dynamics in two‐kidney Goldblatt hypertension. Circ Res. 1983;53(5):592‐602. [DOI] [PubMed] [Google Scholar]
- 9. Ichikawa I, Pfeffer JM, Pfeffer MA, Hostetter TH, Brenner BM. Role of angiotensin II in the altered renal function of congestive heart failure. Circ Res. 1984;55(5):669‐675. [DOI] [PubMed] [Google Scholar]
- 10. Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89(1):493‐498. [DOI] [PubMed] [Google Scholar]
- 11. Schlueter W, Keilani T, Batlle DC. Tissue renin angiotensin systems: theoretical implications for the development of hyperkalemia using angiotensin‐converting enzyme inhibitors. Am J Med Sci. 1994;307(Suppl 1):S81‐86. [PubMed] [Google Scholar]
- 12. Thomas MC, Pickering RJ, Tsorotes D, et al. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107(7):888‐897. [DOI] [PubMed] [Google Scholar]
- 13. Sifi A, Adi‐Bessalem S, Laraba‐Djebari F. Role of angiotensin II and angiotensin type‐1 receptor in scorpion venom‐induced cardiac and aortic tissue inflammation. Exp Mol Pathol. 2017;102(1):32‐40. [DOI] [PubMed] [Google Scholar]
- 14. Xu Z, Li W, Han J, et al. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein‐2 (MD2). Sci Rep. 2017;7:44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Yang J, Yu X, Cheng S, Gan H, Xia Y. Angiotensin II‐induced early and Late Inflammatory responses through NOXs and MAPK pathways. Inflammation. 2017;40(1):154‐165. [DOI] [PubMed] [Google Scholar]
- 16. Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179(2):137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Candido R, Jandeleit‐Dahm KA, Cao Z, et al. Prevention of accelerated atherosclerosis by angiotensin‐converting enzyme inhibition in diabetic apolipoprotein E‐deficient mice. Circulation. 2002;106(2):246‐253. [DOI] [PubMed] [Google Scholar]
- 18. Wysocki J, Ortiz‐Melo DI, Mattocks NK, et al. ACE2 deficiency increases NADPH‐mediated oxidative stress in the kidney. Physiol Rep. 2014;2(3):e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24(9):471‐478. [DOI] [PubMed] [Google Scholar]
- 20. Batlle D, Wysocki J, Soler MJ, Ranganath K. Angiotensin‐converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81(6):520‐528. [DOI] [PubMed] [Google Scholar]
- 21. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238‐33243. [DOI] [PubMed] [Google Scholar]
- 22. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):E1‐9. [DOI] [PubMed] [Google Scholar]
- 23. Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin‐converting enzyme‐related carboxypeptidase. J Biol Chem. 2002;277(17):14838‐14843. [DOI] [PubMed] [Google Scholar]
- 24. Guy JL, Jackson RM, Acharya KR, Sturrock ED, Hooper NM, Turner AJ. Angiotensin‐converting enzyme‐2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42(45):13185‐13192. [DOI] [PubMed] [Google Scholar]
- 25. Wysocki J, Ye M, Rodriguez E, et al. Targeting the degradation of angiotensin II with recombinant angiotensin‐converting enzyme 2: prevention of angiotensin II‐dependent hypertension. Hypertension. 2010;55(1):90‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin‐(1–7). Hypertension. 1997;30(3 Pt 2):535‐541. [DOI] [PubMed] [Google Scholar]
- 27. Ferrario CM. ACE2: more of Ang‐(1–7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marquez A, Batlle D. Angiotensin‐(1–7) for diabetic kidney disease: better than an angiotensin‐converting enzyme inhibitor alone? Kidney Int. 2019;96(4):815‐817. [DOI] [PubMed] [Google Scholar]
- 29. Cassis P, Locatelli M, Corna D, et al. Addition of cyclic angiotensin‐(1–7) to angiotensin‐converting enzyme inhibitor therapy has a positive add‐on effect in experimental diabetic nephropathy. Kidney Int. 2019;96(4):906‐917. [DOI] [PubMed] [Google Scholar]
- 30. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin‐converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maier C, Schadock I, Haber PK, et al. Prolylcarboxypeptidase deficiency is associated with increased blood pressure, glomerular lesions, and cardiac dysfunction independent of altered circulating and cardiac angiotensin II. J Mol Med (Berl). 2017;95(5):473‐486. [DOI] [PubMed] [Google Scholar]
- 32. Velez JC. Prolyl carboxypeptidase: a forgotten kidney angiotensinase. Focus on "Identification of prolyl carboxypeptidase as an alternative enzyme for processing of renal angiotensin II using mass spectrometry". Am J Physiol Cell Physiol. 2013;304(10):C939‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serfozo P, Wysocki J, Gulua G, et al. Ang II (Angiotensin II) Conversion to Angiotensin‐(1–7) in the Circulation Is POP (Prolyloligopeptidase)‐Dependent and ACE2 (Angiotensin‐Converting Enzyme 2)‐Independent. Hypertension. 2020;75(1):173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the Angiotensin converting enzyme 2‐Angiotensin (1–7)‐MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Frontiers in endocrinology. 2014;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19(6_pt_2):692‐696. [DOI] [PubMed] [Google Scholar]
- 36. Domenig O, Manzel A, Grobe N, et al. Neprilysin is a mediator of alternative renin‐angiotensin‐system activation in the murine and human kidney. Sci Rep. 2016;6:33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharp S, Poglitsch M, Zilla P, Davies NH, Sturrock ED. Pharmacodynamic effects of C‐domain‐specific ACE inhibitors on the renin‐angiotensin system in myocardial infarcted rats. JRAAS. 2015;16(4):1149‐1158. [DOI] [PubMed] [Google Scholar]
- 38. Jackman HL, Massad MG, Sekosan M, et al. Angiotensin 1–9 and 1–7 release in human heart: role of cathepsin A. Hypertension. 2002;39(5):976‐981. [DOI] [PubMed] [Google Scholar]
- 39. Flores‐Munoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. Angiotensin1‐9 antagonises pro‐hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol. 2011;589(Pt 4):939‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin‐converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77(2):301‐308. [DOI] [PubMed] [Google Scholar]
- 41. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y,Zuo W. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCov. bioRxiv, 2020; 2020:2026, 2001.919985. [Google Scholar]
- 43. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Batlle D, Wysocki J, Satchell K. Soluble angiotensin‐converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond). 2020;134(5):543‐545. [DOI] [PubMed] [Google Scholar]
- 46. Camargo SMR, Singer D, Makrides V, et al. Tissue‐specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136(3):872‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clarke NE, Fisher MJ, Porter KE, Lambert DW, Turner AJ. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS One. 2012;7(4):e34747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1–7 Axis of the renin‐angiotensin system in heart failure. Circ Res. 2016;118(8):1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilsdorf T, Gainer JV, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin‐(1–7) does not affect vasodilator or TPA responses to bradykinin in human forearm. Hypertension. 2001;37(4):1136‐1140. [DOI] [PubMed] [Google Scholar]
- 50. Durand MJ, Zinkevich NS, Riedel M, et al. Vascular actions of angiotensin 1–7 in the human microcirculation: Novel role for Telomerase. Arterioscler Thromb Vasc Biol. 2016;36(6):1254‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burrell LM, Gayed D, Griggs K, Patel SK, Velkoska E. Adverse cardiac effects of exogenous angiotensin 1–7 in rats with subtotal nephrectomy are prevented by ACE inhibition. PLoS One. 2017;12(2):e0171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin‐(1–7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond). 2011;120(8):335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huentelman MJ, Grobe JL, Vazquez J, et al. Protection from angiotensin II‐induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90(5):783‐790. [DOI] [PubMed] [Google Scholar]
- 54. Díez‐Freire C, Vázquez J, Correa de Adjounian MF, et al. ACE2 gene transfer attenuates hypertension‐linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 55. Donoghue M, Wakimoto H, Maguire CT, et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35(9):1043‐1053. [DOI] [PubMed] [Google Scholar]
- 56. Yamazato M, Yamazato Y, Sun C, Diez‐Freire C, Raizada MK. Overexpression of angiotensin‐converting enzyme 2 in the rostral ventrolateral medulla causes long‐term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49(4):926‐931. [DOI] [PubMed] [Google Scholar]
- 57. Morrell NW, Morris KG, Stenmark KR. Role of angiotensin‐converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269(4 Pt 2):H1186‐1194. [DOI] [PubMed] [Google Scholar]
- 58. Orte C, Polak JM, Haworth SG, Yacoub MH, Morrell NW. Expression of pulmonary vascular angiotensin‐converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol. 2000;192(3):379‐384. [DOI] [PubMed] [Google Scholar]
- 59. Yamazato Y, Ferreira AJ, Hong K‐H, et al. Prevention of pulmonary hypertension by Angiotensin‐converting enzyme 2 gene transfer. Hypertension. 2009;54(2):365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feng Y, Xia H, Cai Y, et al. Brain‐selective overexpression of human Angiotensin‐converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106(2):373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang T, Gao L, Lu J, Zhang YD. ACE2‐Ang‐(1–7)‐Mas axis in brain: A potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol. 2013;11(2):209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nadarajah R, Milagres R, Dilauro M, et al. Podocyte‐specific overexpression of human angiotensin‐converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82(3):292‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu CX, Hu Q, Wang Y, et al. Angiotensin‐converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17(1–2):59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28(12):1287‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wysocki J, Ye M, Khattab AM, et al. Angiotensin‐converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int. 2017;91(6):1336‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang F, Yang J, Zhang Y, et al. Angiotensin‐converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11(7):413‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Towler P, Staker B, Prasad SG, et al. ACE2 X‐ray structures reveal a large hinge‐bending motion important for inhibitor binding and catalysis. J Biol Chem. 2004;279(17):17996‐18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Oudit GY, Penninger JM. Recombinant human angiotensin‐converting enzyme 2 as a new renin‐angiotensin system peptidase for heart failure therapy. Curr Heart Fail Rep. 2011;8(3):176‐183. [DOI] [PubMed] [Google Scholar]
- 70. Zhong J, Basu R, Guo D, et al. Angiotensin‐converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(7):717‐728, 718 p following 728. [DOI] [PubMed] [Google Scholar]
- 71. Haschke M, Schuster M, Poglitsch M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin‐converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52(9):783‐792. [DOI] [PubMed] [Google Scholar]
- 72. Treml B, Neu N, Kleinsasser A, et al. Recombinant angiotensin‐converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide‐induced lung injury in piglets. Crit Care Med. 2010;38(2):596‐601. [DOI] [PubMed] [Google Scholar]
- 73. Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hemnes AR, Rathinasabapathy A, Austin EA, et al. A potential therapeutic role for angiotensin‐converting enzyme 2 in human pulmonary arterial hypertension. Europ Resp J. 2018;51(6):1702638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ye M, Wysocki J, Gonzalez‐Pacheco FR, et al. Murine recombinant angiotensin‐converting enzyme 2: effect on angiotensin II‐dependent hypertension and distinctive angiotensin‐converting enzyme 2 inhibitor characteristics on rodent and human angiotensin‐converting enzyme 2. Hypertension. 2012;60(3):730‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Poglitsch M, Domenig O, Schwager C, et al. Recombinant expression and characterization of human and murine ACE2: Species‐specific activation of the alternative Renin‐angiotensin‐system. Int J Hypertens. 2012;2012:428950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wysocki J, Garcia‐Halpin L, Ye M, et al. Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol. 2013;305(4):F600‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haber PK, Ye M, Wysocki J, Maier C, Haque SK, Batlle D. Angiotensin‐converting enzyme 2‐independent action of presumed angiotensin‐converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertension. 2014;63(4):774‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu P, Wysocki J, Souma T, et al. Novel ACE2‐Fc chimeric fusion provides long‐lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney Int. 2018;94(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 80. Wysocki J, Schulze A, Novel BD. Variants of angiotensin converting Enzyme‐2 of shorter molecular size to target the kidney renin Angiotensin. System. Biomolecules. 2019;9(12):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fan R, Min H, Hong X, et al. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio‐sorbent with superior adsorption capacities for gold and palladium. J Hazard Mater. 2019;364:780‐790. [DOI] [PubMed] [Google Scholar]
- 82. Wysocki J, Goodling A, Burgaya M, et al. Urine RAS components in mice and people with type 1 diabetes and chronic kidney disease. Am J Physiol Renal Physiol. 2017;313(2):F487‐F494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thelle K, Christensen EI, Vorum H, Orskov H, Birn H. Characterization of proteinuria and tubular protein uptake in a new model of oral L‐lysine administration in rats. Kidney Int. 2006;69(8):1333‐1340. [DOI] [PubMed] [Google Scholar]
- 84. Tang J, Wysocki J, Ye M, et al. Urinary renin in patients and mice with diabetic kidney disease. Hypertension. 2019;74(1):83‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hernández Prada JA, Ferreira AJ, Katovich MJ, et al. Structure‐based identification of small‐molecule angiotensin‐converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51(5):1312‐1317. [DOI] [PubMed] [Google Scholar]
- 86. Kuriakose S, Muleme HM, Onyilagha C, Singh R, Jia P, Uzonna JE. Diminazene aceturate (Berenil) modulates the host cellular and inflammatory responses to Trypanosoma congolense infection. PLoS One. 2012;7(11):e48696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kulemina LV, Ostrov DA. Prediction of off‐target effects on angiotensin‐converting enzyme 2. J Biomol Screen. 2011;16(8):878‐885. [DOI] [PubMed] [Google Scholar]
- 88. Lima AM, Xavier CH, Ferreira AJ, et al. Activation of angiotensin‐converting enzyme 2/angiotensin‐(1–7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol. 2013;305(7):H1057‐1067. [DOI] [PubMed] [Google Scholar]
- 89. Ferreira AJ, Shenoy V, Yamazato Y, et al. Evidence for angiotensin‐converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(11):1048‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rigatto K, Casali KR, Shenoy V, Katovich MJ, Raizada MK. Diminazene aceturate improves autonomic modulation in pulmonary hypertension. Eur J Pharmacol. 2013;713(1–3):89‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shenoy V, Gjymishka A, Jarajapu YP, et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med. 2013;187(6):648‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Foureaux G, Nogueira JC, Nogueira BS, et al. Antiglaucomatous effects of the activation of intrinsic Angiotensin‐converting enzyme 2. Invest Ophthalmol Vis Sci. 2013;54(6):4296‐4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qi YanFei, Zhang J, Cole‐Jeffrey CT, et al. Diminazene aceturate enhances angiotensin‐converting Enzyme 2 activity and attenuates ischemia‐induced cardiac pathophysiology. Hypertension. 2013;62(4):746‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ferreira AJ, Shenoy V, Qi Y, et al. Angiotensin‐converting enzyme 2 activation protects against hypertension‐induced cardiac fibrosis involving extracellular signal‐regulated kinases. Exp Physiol. 2011;96(3):287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fraga‐Silva RA, Costa‐Fraga FP, Murça TM, et al. Angiotensin‐converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61(6):1233‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fraga‐Silva RA, Sorg BS, Wankhede M, et al. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16(5–6):210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lima AM, Xavier CH, Ferreira AJ, et al. Activation of angiotensin‐converting enzyme 2/Angiotensin‐(1–7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol. 2013;305(7):H1057‐H1067 [DOI] [PubMed] [Google Scholar]
- 98. Murca TM, Almeida TC, Raizada MK, Ferreira AJ. Chronic activation of endogenous angiotensin‐converting enzyme 2 protects diabetic rats from cardiovascular autonomic dysfunction. Exp Physiol. 2012;97(6):699‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Murça TM, Moraes PL, Capuruço CAB, et al. Oral administration of an angiotensin‐converting enzyme 2 activator ameliorates diabetes‐induced cardiac dysfunction. Regul Pept. 2012;177(1–3):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Foureaux G, Nogueira JC, Nogueira BS, et al. Antiglaucomatous effects of the activation of intrinsic angiotensin‐converting enzyme 2. Invest Ophthalmol Vis Sci. 2013;54(6):4296‐4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Qi YF, Zhang J, Cole‐Jeffrey CT, et al. Diminazene aceturate enhances angiotensin‐converting enzyme 2 activity and attenuates ischemia‐induced cardiac pathophysiology. Hypertension. 2013;62(4):746‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Goru SK, Kadakol A, Malek V, Pandey A, Sharma N, Gaikwad AB. Diminazene aceturate prevents nephropathy by increasing glomerular ACE2 and AT2 receptor expression in a rat model of type1 diabetes. Br J Pharmacol. 2017;174(18):3118‐3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liao W, Bhullar KS, Chakrabarti S, Davidge ST, Wu J. Egg white‐derived tripeptide IRW (Ile‐Arg‐Trp) is an activator of angiotensin converting Enzyme 2. J Agric Food Chem. 2018;66(43):11330‐11336. [DOI] [PubMed] [Google Scholar]
- 104. Oudit GY, Liu GC, Zhong J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59(2):529‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin‐converting enzyme 2 and Angiotensin‐converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067‐3075. [DOI] [PubMed] [Google Scholar]
- 106. Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51(4):613‐623. [DOI] [PubMed] [Google Scholar]
- 107. Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74(12):1610‐1616. [DOI] [PubMed] [Google Scholar]
- 108. Bae EH, Fang F, Williams VR, et al. Murine recombinant angiotensin‐converting enzyme 2 attenuates kidney injury in experimental Alport syndrome. Kidney Int. 2017;91(6):1347‐1361. [DOI] [PubMed] [Google Scholar]
- 109. Ross MJ, Nangaku M. ACE2 as therapy for glomerular disease: the devil is in the detail. Kidney Int. 2017;91(6):1269‐1271. [DOI] [PubMed] [Google Scholar]
- 110. Siragy HM, Carey RM. Role of the intrarenal renin‐angiotensin‐aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31(6):541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Carey RM, Siragy HM. The intrarenal renin‐angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14(6):274‐281. [DOI] [PubMed] [Google Scholar]
- 112. Gurley SB, Coffman TM. The renin‐angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27(2):144‐152. [DOI] [PubMed] [Google Scholar]
- 113. Anderson S, Jung FF, Ingelfinger JR. Renal renin‐angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993;265(4 Pt 2):F477‐486. [DOI] [PubMed] [Google Scholar]
- 114. Kelly TN, Raj D, Rahman M, et al. The role of renin‐angiotensin‐aldosterone system genes in the progression of chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrol Dial Transplant. 2015;30(10):1711‐1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ba Aqeel SH, Sanchez A, Batlle D. Angiotensinogen as a biomarker of acute kidney injury. Clin Kidney J. 2017;10(6):759‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998;274(1 Pt 2):F79‐90. [DOI] [PubMed] [Google Scholar]
- 117. da Silveira KD, Pompermayer Bosco KS, Diniz LRL, et al. ACE2‐angiotensin‐(1–7)‐Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond). 2010;119(9):385‐394. [DOI] [PubMed] [Google Scholar]
- 118. Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol. 2000;279(4):F636‐645. [DOI] [PubMed] [Google Scholar]
- 119. Sharma N, Anders HJ, Gaikwad AB. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed Pharmacother. 2018;110:764‐774. [DOI] [PubMed] [Google Scholar]
- 120. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756‐766. [DOI] [PubMed] [Google Scholar]
- 121. Raizada V, Skipper B, Luo W, Griffith J. Intracardiac and intrarenal renin‐angiotensin systems: Mechanisms of cardiovascular and renal effects. J Investig Med. 2007;55(7):341‐359. [DOI] [PubMed] [Google Scholar]
- 122. Mackie FE, Campbell DJ, Meyer TW. Intrarenal angiotensin and bradykinin peptide levels in the remnant kidney model of renal insufficiency. Kidney Int. 2001;59(4):1458‐1465. [DOI] [PubMed] [Google Scholar]
- 123. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zou Z, Yan Y, Shu Y, et al. Angiotensin‐converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gu H, Xie Z, Li T, et al. Angiotensin‐converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6:19840. [DOI] [PMC free article] [PubMed] [Google Scholar]


