1.
The SARS‐CoV‐2 infection is spreading fast and represents a menacing threat to global health. Evidence accrued so far highlights that diabetes mellitus ranked second, as the most represented comorbidity in infected patients. 1 Data from Chinese populations showed that the prevalence of diabetes in patients with SARS‐CoV‐2 infection was 10.3%, while its nationwide prevalence was 10.9%, hence suggesting that diabetes may not increase the risk of new coronavirus infection. 1 However, a phenome‐wide Mendelian randomization study linked diabetes to increased lung expression of ACE2, the viral cellular receptor; diabetes is also associated with elevated circulating levels of furin and other proteases that facilitate SARS‐CoV‐2 fusion with host cells by cleaving its spike protein. 2 On the other hand, diabetes has been consistently associated with an unfavourable course of the infection. 1 A recent meta‐analysis of eight observational studies showed that diabetic patients with SARS‐CoV‐2 infection are burdened by significantly higher odds of ICU admission (OR 2.79, P < 0.0001) and mortality (OR 3.21, P < 0.0001). 3 Notably, severe obesity, often coexisting with diabetes, is also highly predictive of hospitalization risk. 2
Similarly, in the past years, diabetes was among the most frequent comorbidities in subjects infected with MERS‐CoV and SARS‐CoV, also then associated with a 3‐fold increased mortality. 4 During the SARS epidemic, plasma glucose levels were regarded as independent predictors of increased morbidity and mortality, while these data are still largely unavailable for SARS‐CoV‐2 infected patients. 2 , 4 It is also unclear if the worse prognosis of COVID‐19 in diabetes is due to higher prevalence of comorbidities; nevertheless, as information piles up, an interaction between the consequences of SARS‐CoV‐2 infection and diabetes complications seems to emerge (Figure 1).
FIGURE 1.
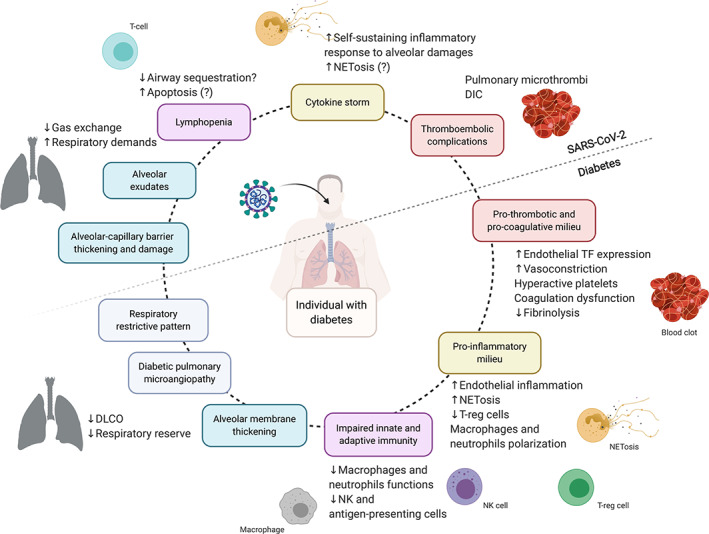
Interaction between SARS‐CoV‐2 infection and diabetes. Diabetes is associated with a reduced respiratory reserve due to alveolar membrane thickening, pulmonary microangiopathy, and restrictive respiratory features. Severe forms of SARS‐CoV‐2 infection could challenge the diabetic lung by generating further thickening of the alveolar‐capillary barrier and alveolar exudates. Virus‐induced lymphopenia might be added to a pre‐existing immune system dysregulation. The pro‐inflammatory, pro‐thrombotic and pro‐coagulative milieu induced by chronic hyperglycaemia might facilitate and enhance the inflammatory response following SARS‐CoV‐2 infection and its thromboembolic complications. DLCO, diffusing capacity of the lung for carbon monoxide; T‐reg cells, regulatory T cells; NETosis, release and activation of neutrophil extracellular traps; TF, tissue factor; DIC, disseminated intravascular coagulation
Li et al hypothesized that, in mild cases of COVID‐19, lung‐resident macrophages were able to orchestrate the immune response to SARS‐CoV‐2 infection, curbing viral replication and diffusion to other susceptible organs. 5 In contrast, patients affected from severe forms of COVID‐19 could not limit the infection to the respiratory tract and often experienced viral sepsis. 5 The critically damaged alveolar‐capillary barrier and large alveolar exudates are powerful triggers for secretion of pro‐inflammatory cytokines and chemotaxis of scavenging cells, initiating the infamous “cytokine storm” thought to be responsible for most of the unfavourable outcomes of COVID‐19. 5 SARS‐CoV‐2 infection is also typically associated with reduction of peripheral T and B lymphocytes, which are essential for cell‐ and antibody‐mediated responses to viral infection. Interestingly, the degree of lymphopenia appears to be positively correlated with disease severity and secondary bacterial infection. 5
Resembling the duplicity of SARS‐CoV‐2, diabetes is also associated both with immune system deficiency and aberrant inflammation. 4 Since diabetes and uncontrolled hyperglycaemia have been linked to impaired functions of macrophages and neutrophils, it can be hypothesized that this may allow the infection to spread out of the respiratory tract. 6 Moreover, diabetes‐related impairment of NK cells and antigen‐presenting cells could also concur to delay the development of adaptive immunity and facilitate bacterial superinfection. 7 Specifically, in vitro studies demonstrated that exposure to hyperglycaemia promoted influenza virus infection and replication in pneumocytes. 6 On the other hand, reduction of regulatory T cells and polarization of macrophages and neutrophils towards a pro‐inflammatory phenotype accounts for excess cytokine release and establishment of a pro‐inflammatory milieu in diabetic patients. 7
Recently, Barnes et al suggested that COVID‐19, 8 just like hyperglycaemia, 9 might induce abnormal production of neutrophil extracellular traps (NETs). NETosis is a neutrophil‐specific strategy to kill extracellular pathogens, consisting in the release of a decondensed chromatin web beaded with granular antimicrobial enzymes. 9 Abnormal NETosis has been associated with acute respiratory distress syndrome (ARDS) development following a variety of triggers, including influenza virus, and can induce macrophage IL‐1β secretion, which in turn stimulates NETs formation; this originates a self‐sustaining loop that can induce aberrant inflammation and diffuse thrombosis and precipitate respiratory function. 8 Interestingly, increased NETosis has been detected also in patients with diabetic retinopathy 9 and other complications. Moreover, most of the non‐survivor COVID‐19 patients experienced disseminated intravascular coagulation, often heralded by increased levels of D‐Dimer and fibrin degradation products. 5 Diabetes could fuel this process, as it is characterized by a pro‐thrombotic and pro‐coagulative environment. 6
The SARS‐CoV‐2 infection mainly targets the lungs and can provoke acute hypoxemic respiratory failure in susceptible patients. 1 Despite this is often neglected, diabetes can also directly affect pulmonary architecture and function. 10 Autoptic studies have described structural abnormalities, such as thickening and fibrosis of the alveolar basement membrane, resembling those observed in diabetic retina and kidneys. 10 Diabetic patients frequently displayed a restrictive respiratory pattern, probably due to reduced muscle strength and endurance, excess body fat, and AGE‐mediated accumulation of collagen in lungs and chest walls. 10 These features may compromise the pulmonary reserve in diabetic individuals, 10 making conditions with increased respiratory demand, such as COVID‐19, hardly manageable. Moreover, diabetic patients showed lower pulmonary microvascular distensibility and reduced ability to recruit new capillary beds, especially in the presence of other known microvascular complications. 10 , 11 These features were also associated with reduced right ventricle function, pulmonary hypertension, and lower exercise capacity. 10 Additionally, the diabetic lung exhibited a reduced diffusion capacity for carbon monoxide (DLCO), both due to alveolar membrane thickening and pulmonary microangiopathy. 10 , 12 Aging could facilitate diabetes‐induced pulmonary damages. 12 Consistently, post‐mortem findings of SARS‐CoV‐2‐infected lungs show various degrees of diffuse alveolar damage, with interstitial thickening and mononuclear cellular infiltration. 13 The negative outcome of diabetic individuals with COVID‐19 could thus result from the unfortunate interaction of a unique viral infection with a specifically compromised pulmonary tissue.
2. CONFLICT OF INTEREST
The authors declare that they have no financial competing interests.
AUTHOR CONTRIBUTIONS
I.C. and F.G. contributed equally to the final manuscript.
REFERENCES
- 1. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest. 2020;43:867‐869. 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muniyappa R, Gubbi S. COVID‐19 pandemic, corona viruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736‐E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID‐19 infection are at higher risk of ICU admission and poor short‐term outcome. J Clin Virol. 2020;127:104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011. 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Liu L, Zhang D, et al. Hypothesis SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517‐1520. 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID‐19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res. 2018;2018:7457269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A, et al. Targeting potential drivers of COVID‐19: neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. 10.1084/jem.e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Zhou X, Yin Y, Mai Y, Wang D, Zhang X. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase‐dependent pathway in diabetic retinopathy. Front Immunol. 2019;9:3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mauricio D, Alonso N, Gratacòs M. Chronic diabetes complications: the need to move beyond classical concepts. Trends Endocrinol Metab. 2020;31(4):287‐295. [DOI] [PubMed] [Google Scholar]
- 11. Roberts TJ, Burns AT, MacIsaac RJ, MacIsaac AI, Prior DL, La Gerche A. Diagnosis and significance of pulmonary microvascular disease in diabetes. Diabetes Care. 2018;41:854‐861. [DOI] [PubMed] [Google Scholar]
- 12. Fuso L, Pitocco D, Antonelli‐Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metab Res Rev. 2019;35(6):e3159. [DOI] [PubMed] [Google Scholar]
- 13. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through post‐mortem core biopsies. Mod Pathol. 2020;1‐8. 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


