Abstract
The coronavirus disease 2019 (COVID‐19) pandemic has caused respiratory failure and associated mortality in numbers that have overwhelmed global health systems. Thrombotic coagulopathy is present in nearly three quarters of patients with COVID‐19 admitted to the intensive care unit, and both the clinical picture and pathologic findings are consistent with microvascular occlusive phenomena being a major contributor to their unique form of respiratory failure. Numerous studies are ongoing focusing on anticytokine therapies, antibiotics, and antiviral agents, but none to date have focused on treating the underlying thrombotic coagulopathy in an effort to improve respiratory failure in COVID‐19. There are animal data and a previous human trial demonstrating a survival advantage with fibrinolytic therapy to treat acute respiratory distress syndrome. Here, we review the extant and emerging literature on the relationship between thrombotic coagulopathy and pulmonary failure in the context of COVID‐19 and present the scientific rationale for consideration of targeting the coagulation and fibrinolytic systems to improve pulmonary function in these patients.
Keywords: acute respiratory distress syndrome, COVID‐19, fibrinolysis, pulmonary failure, tissue plasminogen activator
Essentials.
Coronavirus disease 2019 (COVID‐19) has caused thrombotic coagulopathy and respiratory failure in unprecedented numbers.
Pulmonary microvascular thrombosis is particularly prominent in COVID‐19 respiratory failure.
Animal and limited human data support a role for fibrinolytic therapy in respiratory failure.
Urgent study of fibrinolytic therapy is needed. A phase 2a trial is pending (NCT 04357730).
1. INTRODUCTION
As the coronavirus disease 2019 (COVID‐19) pandemic accelerates, cases have grown exponentially around the world. Other countries’ experience suggests that 5%‐16% of in‐patients with COVID‐19 will undergo prolonged intensive care, 1 , 2 , 3 with 50%‐70% needing mechanical ventilation (MV), 3 , 4 , 5 threatening to overwhelm hospital capacity. 6
While COVID‐19 overall mortality likely ranges from 1% to 5%, this is much higher in patients with COVID‐19–induced acute respiratory distress syndrome (ARDS) (22%‐64%). 3 , 4 , 7 There are currently few proven ARDS therapies other than low‐tidal‐volume 8 and prone‐positioning 9 MV. Most current trials on clinicaltrials.gov for COVID‐19–induced ARDS aim at modulating the inflammatory response. Sarilumab and tocilizumab, which block interleukin‐6 effects, are being tested in randomized controlled trials for patients hospitalized with severe COVID‐19 (NCT04317092 9 ). The World Health Organization international trial SOLIDARITY will test remdesivir, chloroquine plus hydroxychloroquine, lopinavir plus ritonavir, and lopinavir plus ritonavir and interferon‐β. However, studies targeting the coagulation system, which is intrinsically intertwined with the inflammatory response, are lacking. 10 , 11 , 12 , 13 , 14
2. FIBRINOLYSIS, ARDS, AND THE POSSIBLE ROLE OF FIBRINOLYTIC THERAPY IN COVID‐19
Our group has shown that low fibrinolysis is associated with ARDS, 15 , 16 , 17 , 18 , 19 and patients with COVID‐19 in the intensive care unit (ICU) have now been shown on thromboelastography to universally have lower levels of fibrinolysis than the reference population. 20 Over the past decades, studies have demonstrated the systemic and local effects of dysfunctional coagulation, specifically related to fibrin, in ARDS. 11 , 13 , 14 , 21 , 22 ARDS, regardless of cause, is associated with fibrin deposition in air spaces and fibrin‐platelet microthrombi in the pulmonary vasculature, 23 , 24 , 25 which is also consistently observed in the lung microvasculature of patients with COVID‐19. 26 , 27 , 28 This pathologic fibrin deposition reflects a dysfunctional clotting system, with enhanced clot formation and propagation as well as fibrinolysis suppression, 29 , 30 , 31 largely due to tissue factor produced by alveolar epithelial cells and macrophages, 32 and high levels of plasminogen activator inhibitor‐1 (PAI‐1) produced by endothelial cells or activated platelets. 33 , 34 Consistent with this, prothrombin time prolongation, elevated D‐dimer and fibrin degradation products, and uniquely elevated fibrinogen levels have been reported in severely ill patients with COVID‐19, particularly in nonsurvivors. 3 , 4 , 20 , 35 , 36 , 37 , 38 Similar findings have been observed in sepsis, 29 , 39 endotoxemia, 40 and extensive tissue disruption, 18 in which early activation of coagulation and fibrinolysis is followed by late fibrinolytic shutdown and endothelial dysfunction. It is also consistent with an initial viral infection of airway epithelial cells, with later spread to endothelial cells, which has now been shown to occur in COVID‐19, 41 both of which express the receptor protein for the virus, angiotensin‐converting‐enzyme‐2 (ACE2). 42 Furthermore, it has now also been shown that critically ill patients with COVID‐19 universally demonstrate hypercoagulable findings on viscoelastic assays relative to the reference population, with shortened reaction time, increased α‐angle, increased maximal amplitude, and in virtually all cases a reduced level of fibrinolysis on thromboelastography. 20
Targeting the coagulation and fibrinolytic systems to improve ARDS and associated pulmonary clot formation syndromes has been described 34 , 43 , 44 , 45 , 46 , 47 and tested in animal models, 48 , 49 , 50 , 51 and in light of the mounting findings in COVID‐19, as described above, may also have a role in the management of COVID‐19 respiratory failure. In 2001, Hardaway and colleagues described a small, noncontrolled human trial in severe ARDS, showing that uro/streptokinase led to remarkable improvement in oxygen requirements without bleeding events. 52 Tissue‐type plasminogen activator (t‐PA) is a more modern fibrinolytic approach with higher clot lysis efficacy without increased bleeding risk. A meta‐analysis of acute lung injury in animals showed that, compared to controls, t‐PA improved survival, arterial pO2 and pCO2 better than either urokinase plasminogen activator or plasmin, although none of the studies included viral‐induced ARDS. 50 In other studies, intra‐airway delivered t‐PA improved survival and morbidity associated with acute plastic bronchitis crisis, in which intra‐airway clotting occurs. 46 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 Both nebulized and direct instillation of t‐PA into the airways via bronchoscopy have been used off‐label to treat fibrin airway casts. 61 In a lethal animal model of both severe pulmonary microvascular thrombi and severe bronchial fibrin casts, treatment with airway t‐PA resulted in improved survival, dissolved airway casts, and normalized pO2 and pCO2. 62 , 63 However, the mounting evidence specific to COVID‐19 that shows pulmonary microvascular thrombosis as a predominant finding 26 , 27 , 28 combined with normal lung compliance and high alveolar‐arterial oxygen gradients 64 suggests intravascular delivery may be the more appropriate delivery route, with concern that intra‐airway delivery via intratracheal instillation or nebulized solutions may increase the risk to health care workers by exposing them to infectious airway secretions.
Taken together, the extant data on fibrinolytic therapy in ARDS combined with the thrombotic coagulopathy and clinical findings consistent with pulmonary vascular thrombo‐occlusive disease in COVID‐19 suggest that manipulation of the fibrinolytic system through administration of t‐PA may have a role in the therapy of severe, medically refractory COVID‐19–induced ARDS. Importantly, such an approach is nonexclusive and could be used in patients who have been treated with other experimental agents, including anti–interleukin‐6 receptor blockers and other immune modulators, antibiotics, and antiviral agents.
3. RISK CONSIDERATIONS FOR FIBRINOLYTIC THERAPY IN COVID‐19 ARDS
The main risk if fibrinolytic therapy were considered for treatment of severe, medically refractory hypoxemia in COVID‐19 respiratory failure is bleeding. The bleeding risk can be estimated from its use in myocardial infarction (MI) and submassive pulmonary embolism. In the largest available prospectively collected data set of intravenous alteplase for non–stroke indication (GUSTO [Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries] trial; MI), the risk of hemorrhagic stroke was 0.7% and severe/life‐threatening bleeding was 0.4% in the group that received 50 mg of alteplase over 90 minutes followed immediately by a 5000 U bolus of intravenous unfractionated heparin and a therapeutic heparin drip (n = 10 396 patients). 65 In a trial of high‐dose alteplase (100 mg over 2 hours) given concomitant with therapeutic systemic heparinization for submassive pulmonary embolism, the rate of major bleeding was 0.8%, and none of the 118 patients in the alteplase arm of the trial developed a hemorrhagic stroke. 66 These 2 patient groups are expected to be relatively similar to those with severe COVID‐19 illness regarding comorbidities and the absence of active stroke, which increases the risk of hemorrhagic conversion. There are multiple studies that quote higher risks of “major bleeding,” with a commonly referenced meta‐analysis by Chatterjee et al 67 quoting a major bleeding risk with t‐PA in pulmonary embolism as being 9.24% relative to 3.42% for anticoagulation alone. The majority of the patients in this meta‐analysis came from a single study (the PEITHO [Pulmonary Embolism International Thrombolysis] study) that used tenecteplase, 68 which is resistant to PAI‐1 inhibition. 69 Additionally, many of the included studies had no prespecified definition of a major bleeding event, several included studies considered any blood transfusion as a major bleed, and none used hemodynamic parameters or massive transfusion protocol activation as criteria. With respect to COVID‐19 ICU patients on mechanical ventilation where death is as likely as survival, life‐threatening hemorrhage is the most relevant for consideration, which again suggests the GUSTO study of over 40 000 patients including over 10 000 patients in the alteplase bolus plus heparin group is likely the most relevant for risk considerations for fibrinolytic therapy in severe, medically refractory hypoxemic respiratory failure in COVID‐19. 65 Furthermore, given the profound hypercoagulable/thrombotic coagulopathy in the majority of critically ill COVID‐19 patients 20 , 35 , 38 , 70 , 71 where bleeding is quite rare and thrombosis predominates, the risk of systemic fibrinolysis therapy may be even lower. Similarly, while we posit that intravascular delivery of t‐PA would likely be more effective, if intra‐airway t‐PA were to be pursued and effective, the available case reports of t‐PA airway delivery have thus far showed no bleeding events. 46 , 61
In addition to bleeding events, there is also risk specific to the COVID‐19 betacoronavirus itself. Cell entry by this virus requires cleavage of a trimeric spike (S) glycoprotein that protrudes from the viral envelope, generating 2 subunits (S1 and S2), followed by additional cleavage within S2. 72 The S1 subunit provides the receptor binding domain, which binds to ACE2 on the cell surface. 73 , 74 The S2 subunit then drives fusion between the virus and the cell membranes, resulting in viral entry. S protein cleavage at the S1‐S2 junction and within S2 occurs through host cell proteases, primarily transmembrane serine protease 2 (TMPRSS2), at specific arginine‐serine sequences. 72 Plasmin, like TMPRSS2, also favors cleavage between arginine‐serine sequences 75 ; thus, t‐PA administration with plasmin generation could conceivably enhance viral infectivity. However, patients in COVID‐19 terminal stages are likely already massively infected with high viral loads, where the profound hypoxemic respiratory failure resulting from pulmonary microvascular thrombosis and the host inflammatory response to the virus is the imminent threat to life rather than the virus itself. As such, we posit that plasmin activation effects on viral entry or decreased clearance would be minor compared with salvage of pulmonary gas exchange and correction of profound hypoxemic respiratory failure to prevent imminent death by restoring pulmonary microvascular patency.
4. PRACTICAL CONSIDERATIONS IF FIBRINOLYTIC THERAPY WERE CONSIDERED IN COVID‐19 ARDS
In the absence of effective therapies for medically refractory COVID‐19 hypoxemic respiratory failure where critical care physicians have exhausted all available treatment options in a dying patient, patients with prothrombotic presentations, normal lung compliance on the ventilator, and high alveolar‐arterial oxygen gradients could potentially be considered for fibrinolytic therapy; as such, a clinical presentation is suggestive of vascular occlusive disease as a primary cause of hypoxemia. It should be noted that in a disease entity only several months old, such an approach has no pre‐existing controlled trial data and must be considered with extreme caution, although there are now a handful of case reports that so far have demonstrated temporal associations with alteplase administration and improvement of the respiratory status of critically ill patients with COVID‐19 (emphasis: a causal relationship in uncontrolled case reports cannot be inferred) 76 (Poor et al, in review; Barrett et al, in review). The large experience using intravenous t‐PA for strokes, myocardial infarctions, and pulmonary emboli 66 , 77 , 78 may provide a useful guide for its use in COVID‐19 respiratory failure in the absence of prior controlled trial data. Our suggestion in such a situation is to consider an initial intravenous bolus dose of 50 or 100 mg of alteplase over 2 hours, concomitant with, or immediately followed by, systemic anticoagulation with heparin. Multiple sites across the United States have already taken this approach (fibrinolytic therapy) in severe, medically refractory COVID‐19 hypoxemic respiratory failure, and the specifics around the use of heparin vary from fully therapeutic heparin drips (partial thromboplastin time goal 60‐80 seconds) while t‐PA is infusing, to 500 U/h heparin while t‐PA is infusing, followed by full anticoagulation after tPA is finished, to starting therapeutic heparin right after t‐PA finishes infusing with no heparin during the actual tPA administration period. As a rationale for dosing with respect to t‐PA (alteplase), we performed pharmacokinetic simulations on 2 test subjects (75 and 60 kg), and found that the 50‐ and 100‐mg bolus dose regimens would quickly achieve t‐PA plasma concentrations above median PAI‐1 levels in injured patients (200 ng/mL, 4.7 nmol/L) (Moore et al, unpublished; Cardenas et al79) (Figure 1). Importantly, this pharmacokinetic model also supports a redosing strategy at 12 hours or later in transient responders (eg, those that may have rethrombosed due to inadequate anticoagulation, as suspected in the case series observations by Wang et al 76 ), since by this time the plasma levels of t‐PA from the first bolus have fallen below the level of circulating t‐PA in normal patients. 80 A phase II clinical trial will be required to confirm these estimates and is now planned (discussed below). While we believe that intravascular delivery of t‐PA is likely a more appropriate route of administration in COVID‐19 respiratory failure if fibrinolytic therapy were considered, if intra‐airway t‐PA delivery were to be pursued, we suggest 50 mg (or 0.7 mg/kg) of t‐PA instilled into the airways, preferentially by bronchoscopy, followed by repeat dosing every 4‐8 hours as needed for sustained improvement of oxygen requirements. This regimen is based on empiric guidelines for treatment of plastic bronchitis patients at the Children’s Hospital of Colorado, as well as multiple case reports and animal studies. 51 , 54 , 56 , 61 The same exclusion criteria for MI treatment with tPA should apply, with patients maintained on a heparin infusion after t‐PA treatment completion to prevent reaccumulation of fibrin clot in the lung microvasculature. A possible algorithm for consideration of fibrinolytic therapy in severe, medically refractory COVID‐19 respiratory failure is shown in Figure 2, with the key points being: (1) that in the absence of controlled trial data such an approach should be considered only in patients with persistent, refractory hypoxemic respiratory failure despite maximal management strategies; (2) have evidence of a hypercoagulable state; and (3) have normal lung compliance with high alveolar‐arterial oxygen gradients that suggest the patient’s hypoxemia likely has a vascular occlusive component. While this scenario of a hypercoagulable state, normal lung compliance, and high alveolar‐arterial oxygen gradients is seen in the majority of patients with COVID‐19 respiratory failure and microvascular thrombosis is present in the majority of autopsies, the possibility of macrovascular pulmonary embolism is not insignificant 71 , 81 and similarly may improve after fibrinolytic therapy. As discussed above, such an approach involves risk, but such risk in carefully selected patients is likely outweighed by certainty of death in the proposed population and justifies consideration of salvage t‐PA therapy when all other therapeutic options are exhausted. We would encourage all those who are inclined to treat critically ill patients with COVID‐19 with t‐PA for refractory respiratory failure to track the success or failure of this approach and report their clinical outcomes.
Figure 1.
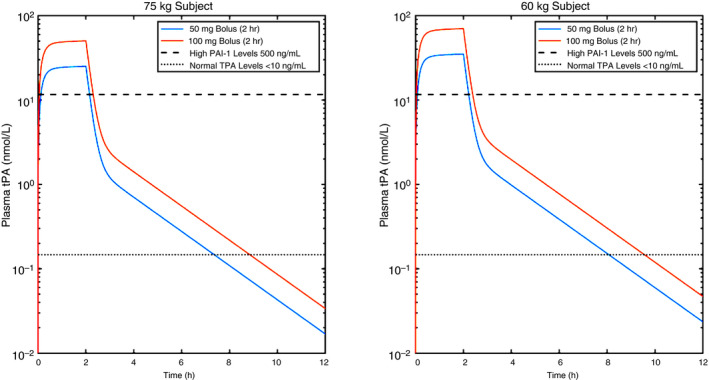
Pharmacokinetic simulations of t‐PA levels over time relative to its native inhibitor (PAI‐1) in patients with COVID‐19 based on various body mass and bolus/maintenance doses of t‐PA. The model assumes a plasma clearance of 8.3 min, and a terminal half‐life of 88 min. 82 PAI‐1, plasminogen activator inhibitor‐1; t‐PA, tissue‐type plasminogen activator
Figure 2.
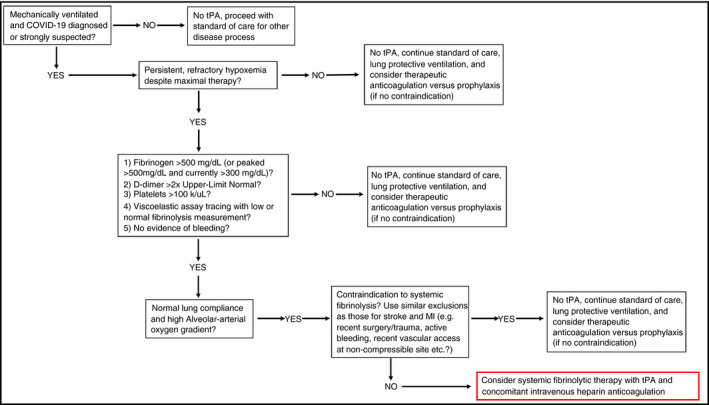
Possible algorithm for consideration of fibrinolytic therapy in severe, medically refractory COVID‐19 respiratory failure. COVID‐19, coronavirus disease 2019; MI, myocardial infarction; t‐PA, tissue‐type plasminogen activator inhibitor‐1
5. SUMMARY AND CONCLUSIONS
In the present COVID‐19 crisis, facing a disease entity that has existed only for several months, physicians (particularly critical care physicians) are faced with large numbers of patients in profound, medically refractory hypoxemic respiratory failure with multiple clinical clues and autopsy reports that suggest a significant pulmonary microvascular thrombotic component. Level 1 evidence from randomized controlled trials for managing COVID‐19 and its associated severe, refractory hypoxemic respiratory failure is months, if not years, away. As such, clinicians facing life‐and‐death situations in critically ill patients with COVID‐19 must treat them using clinical reasoning based on observation of the patient’s physiology, as the standard protocols and best practice “pathways” that modern medicine has become dependent on simply do not exist yet in this emerging and lethal disease. If t‐PA fibrinolytic therapy were used in decompensating patients with no options for escalation of care, and shown to be effective with a greater risk of benefit than harm, such an approach could be rapidly broadened globally due to t‐PA’s availability at most medical centers. While we cannot specifically advocate for its use in a systematic way at this time, and caution against broad implementation of this approach in the absence of controlled trial data, such an approach should at least be known to clinicians treating critically ill patients with COVID‐19 in the event that they have an imminently dying patient meeting the criteria outlined above and in Figure 2, and have exhausted all other options. Formal study of this potential therapy is urgently needed. A phase IIa multicenter randomized controlled trial of alteplase therapy in severe, medically refractory hypoxemic respiratory failure in COVID‐19 is now underway (ClinicalTrials.gov, NCT 04357730).
RELATIONSHIP DISCLOSURE
CDB, HBM, EEM, and MBY have patents pending related to both coagulation/fibrinolysis diagnostics and therapeutic fibrinolytics, and are passive cofounders and hold stock options in Thrombo Therapeutics, Inc. HBM and EEM have received grant support from Haemonetics and Instrumentation Laboratories. EEM holds a grant from Genentech. MBY has previously received a gift of alteplase (t‐PA) from Genentech and owns stock options as a cofounder of Merrimack Pharmaceuticals. JB and JA are cofounders of Applied BioMath and own stock. FH owns stock in Applied BioMath. LAV has received grant support from Genentech. PCM, PKM, AS, DL, RM, and DST report nothing to disclose.
AUTHOR CONTRIBUTION
CDB, HBM, EEM, AS, LAV, and MBY prepared the manuscript with critical input and revisions from DST and DRL. Pharmacokinetics models were generated by JB, FH, and JA.
Barrett CD, Moore HB, Moore EE, et al. Fibrinolytic therapy for refractory COVID‐19 acute respiratory distress syndrome: Scientific rationale and review. Res Pract Thromb Haemost. 2020;4:524–531. 10.1002/rth2.12357
Christopher D. Barrett and Hunter B. Moore are co‐first authors.
Handling Editor: Cihan Ay
Contributor Information
Christopher D. Barrett, @chrisbarrettmd.
Ernest E. Moore, Email: ernest.moore@dhha.org.
Michael B. Yaffe, Email: myaffe@mit.edu.
REFERENCES
- 1. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy. JAMA. 2020;323(16):1545. [DOI] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically Ill patients with COVID‐19 in Washington state. JAMA. 2020;323(16):1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med. 2020;382(21):2049–55. [DOI] [PubMed] [Google Scholar]
- 7. Livingston E, Bucher K. Coronavirus disease 2019 (COVID‐19) in Italy. JAMA. 2020;323(14):1335. [DOI] [PubMed] [Google Scholar]
- 8. Acute Respiratory Distress Syndrome N , Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 9. Guérin C, Reignier J, Richard J‐C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–68. [DOI] [PubMed] [Google Scholar]
- 10. He XL, Liu Z, Xia SY. Vascular endothelial injuries and changes of blood coagulation and fibrinolysis indexes in patients with acute respiratory distress syndrome. Chin Med Sci J. 2004;19(4):252–6. [PubMed] [Google Scholar]
- 11. Hofstra JJ, Haitsma JJ, Juffermans NP, Levi M, Schultz MJ. The role of bronchoalveolar hemostasis in the pathogenesis of acute lung injury. Semin Thromb Hemost. 2008;34(5):475–84. [DOI] [PubMed] [Google Scholar]
- 12. Olson TM, Driscoll DJ, Edwards WD, Puga FJ, Danielson GK. Pulmonary microthrombi. Caveat for successful modified Fontan operation. J Thorac Cardiovasc Surg. 1993;106(4):739–44. [PubMed] [Google Scholar]
- 13. Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S213–20. [DOI] [PubMed] [Google Scholar]
- 14. Idell S, Koenig KB, Fair DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261(4 Pt 1):L240–8. [DOI] [PubMed] [Google Scholar]
- 15. Moore HB, Moore EE. Temporal changes in fibrinolysis following injury. Semin Thromb Hemost. 2020;46(2):189–98. [DOI] [PubMed] [Google Scholar]
- 16. Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore HB, Moore EE, Huebner BR, Dzieciatkowska M, Stettler GR, Nunns GR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83(6):1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222(4):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore HB, Walsh M, Kwaan HC, Medcalf RL. The complexity of trauma‐induced coagulopathy. Semin Thromb Hemost. 2020;46(2):114–5. [DOI] [PubMed] [Google Scholar]
- 20. Panigada MBN, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, et al. Hypercoagulability of COVID‐19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuchs‐Buder T, de Moerloose P, Ricou B, Reber G, Vifian C, Nicod L, et al. Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(1):163–7. [DOI] [PubMed] [Google Scholar]
- 22. Albarello F, Pianura E, Di Stefano F, Cristofaro M, Petrone A, Marchioni L, et al. 2019‐novel coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis. 2020;93:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bone RC, Francis PB, Pierce AK. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med. 1976;61(5):585–9. [DOI] [PubMed] [Google Scholar]
- 24. Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–49. [DOI] [PubMed] [Google Scholar]
- 25. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49. [DOI] [PubMed] [Google Scholar]
- 26. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo W, Yu H‐J, Gou J, Li X, Sun Y, Li J, et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19) Preprint. 2020:1–18. 10.13140/RG.2.2.22934.29762 [DOI]
- 28. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid‐19: the first autopsy series from New Orleans. MedRxiv (preprint). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bastarache JA, Ware LB, Bernard GR. The role of the coagulation cascade in the continuum of sepsis and acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):365–76. [DOI] [PubMed] [Google Scholar]
- 30. Günther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161(2 Pt 1):454–62. [DOI] [PubMed] [Google Scholar]
- 31. Ware LB, Bastarache JA, Wang L. Coagulation and fibrinolysis in human acute lung injury–new therapeutic targets? Keio J Med. 2005;54(3):142–9. [DOI] [PubMed] [Google Scholar]
- 32. Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. 2007;62(7):608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grau GE, de Moerloose P, Bulla O, Lou J, Lei Z, Reber G, et al. Haemostatic properties of human pulmonary and cerebral microvascular endothelial cells. Thromb Haemost. 1997;77(3):585–90. [PubMed] [Google Scholar]
- 34. MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27(6):860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- 36. Wu C, Chen X, Cai Y, Ja X, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: a comment. J Thromb Haemost. 2020. 10.1111/jth.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–45. [DOI] [PubMed] [Google Scholar]
- 40. Ostrowski SR, Berg RM, Windelov NA, Meyer MA, Plovsing RR, Moller K, et al. Discrepant fibrinolytic response in plasma and whole blood during experimental endotoxemia in healthy volunteers. PLoS ONE. 2013;8(3):e59368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varga ZFAJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S329–36. [DOI] [PubMed] [Google Scholar]
- 44. Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia–a review. Crit Care Med. 2006;34(3):871–7. [PubMed] [Google Scholar]
- 45. Ware LB, Camerer E, Welty‐Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L307–11. [DOI] [PubMed] [Google Scholar]
- 46. Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, et al. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32(8):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hardaway RM, Harke H, Williams CH. Fibrinolytic agents: a new approach to the treatment of adult respiratory distress syndrome. Adv Ther. 1994;11(2):43–51. [PubMed] [Google Scholar]
- 48. Hardaway RM, Williams CH, Marvasti M, Farias M, Tseng A, Pinon I, et al. Prevention of adult respiratory distress syndrome with plasminogen activator in pigs. Crit Care Med. 1990;18(12):1413–8. [DOI] [PubMed] [Google Scholar]
- 49. Stringer KA, Hybertson BM, Cho OJ, Cohen Z, Repine JE. Tissue plasminogen activator (tPA) inhibits interleukin‐1 induced acute lung leak. Free Radic Biol Med. 1998;25(2):184–8. [DOI] [PubMed] [Google Scholar]
- 50. Liu C, Ma Y, Su Z, Zhao R, Zhao X, Nie HG, et al. Meta‐analysis of preclinical studies of fibrinolytic therapy for acute lung injury. Front Immunol. 2018;9:1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veress LA, Anderson DR, Hendry‐Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicol Sci. 2015;143(1):178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67(4):377–82. [PubMed] [Google Scholar]
- 53. Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics. 2002;109(4):e67. [DOI] [PubMed] [Google Scholar]
- 54. Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;30(3):352–5. [DOI] [PubMed] [Google Scholar]
- 55. Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long‐term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;6(1):76–8. [DOI] [PubMed] [Google Scholar]
- 56. Colaneri M, Quarti A, Pozzi M, Gasparini S, Carloni I, de Benedictis FM. Management of plastic bronchitis with nebulized tissue plasminogen activator: another brick in the wall. Ital J Pediatr. 2014;40(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brooks K, Caruthers RL, Schumacher KR, Stringer KA. Pharmacotherapy challenges of fontan‐associated plastic bronchitis: a rare pediatric disease. Pharmacotherapy. 2013;33(9):922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Racz J, Mane G, Ford M, Schmidt L, Myers J, Standiford TJ, et al. Immunophenotyping and protein profiling of Fontan‐associated plastic bronchitis airway casts. Ann Am Thorac Soc. 2013;10(2):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lubcke NL, Nussbaum VM, Schroth M. Use of aerosolized tissue plasminogen activator in the treatment of plastic bronchitis. Ann Pharmacother. 2013;47(3):e13. [DOI] [PubMed] [Google Scholar]
- 60. Singhal NR, Da Cruz EM, Nicolarsen J, Schwartz LI, Merritt GR, Barrett C, et al. Perioperative management of shock in two fontan patients with plastic bronchitis. Semin Cardiothorac Vasc Anesth. 2013;17(1):55–60. [DOI] [PubMed] [Google Scholar]
- 61. Gibb E, Blount R, Lewis N, Nielson D, Church G, Jones K, et al. Management of plastic bronchitis with topical tissue‐type plasminogen activator. Pediatrics. 2012;130(2):e446–50. [DOI] [PubMed] [Google Scholar]
- 62. McGraw MD, Osborne CM, Mastej EJ, Di Paola JA, Anderson DR, Holmes WW, et al. Editor's highlight: Pulmonary vascular thrombosis in rats exposed to inhaled sulfur mustard. Toxicol Sci. 2017;159(2):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White CW, Rancourt RC, Veress LA. Sulfur mustard inhalation: mechanisms of injury, alteration of coagulation, and fibrinolytic therapy. Ann NY Acad Sci. 2016;1378(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid‐19 does not lead to a "typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Investigators G . An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673–82. [DOI] [PubMed] [Google Scholar]
- 66. Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W, Management S, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143–50. [DOI] [PubMed] [Google Scholar]
- 67. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial hemorrhage: a meta‐analysis. JAMA. 2014;311(23):2414–21. [DOI] [PubMed] [Google Scholar]
- 68. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer‐Westendorf J, et al. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–11. [DOI] [PubMed] [Google Scholar]
- 69. Davami F, Sardari S, Majidzadeh‐A K, Hemayatkar M, Barkhordari F, Enayati S, et al. A novel variant of t‐PA resistant to plasminogen activator inhibitor‐1; expression in CHO cells based on in silico experiments. BMB Rep. 2011;44(1):34–9. [DOI] [PubMed] [Google Scholar]
- 70. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Klok FA, Kruip M, van der Meer N, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C‐L, Abiona O, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hervio LS, Coombs GS, Bergstrom RC, Trivedi K, Corey DR, Madison EL. Negative selectivity and the evolution of protease cascades: the specificity of plasmin for peptide and protein substrates. Chem Biol. 2000;7(6):443–53. [DOI] [PubMed] [Google Scholar]
- 76. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020. 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaufman C, Kinney T, Quencer K. Practice trends of fibrinogen monitoring in thrombolysis. J Clin Med. 2018;7(5):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014(7):CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. [DOI] [PubMed] [Google Scholar]
- 80. Whitton C, Rigsby P, Longstaff C. A Proposed 1st WHO International Standard for the Measurement of Tissue Plasminogen Activator (tPA) Antigen in Plasma 94/730. https://wwwwhoint/biologicals/expert_committee/BS%202068%20tissue%20plasminogenpdf Accessed on May 15, 2020; 2007.
- 81. Rotzinger DC, Beigelman‐Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID‐19: time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cohen A. Pharmacokinetics of the recombinant thrombolytic agents. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene. Therapy. 1999;11(2):115–23. [DOI] [PubMed] [Google Scholar]


