To the Editor:
Coronavirus disease 2019 (COVID‐19) represents a global health crisis in which personal protective equipment (PPE) has become increasingly limited. Dermatologists are poised to think creatively and use technology, such as teledermatology, to innovate existing workflows and optimize dermatologic care.
We conducted a pilot, retrospective cohort study to evaluate the utility of store‐and‐forward teledermatology during the COVID‐19 pandemic. We included patients seen by the inpatient dermatology consult service at the Ohio State University Wexner Medical Center from 16 March 2020 to 20 March 2020. We used a recently proposed algorithm for how hospital settings can initiate and use telemedicine consultative services during the COVID‐19 pandemic (Figure 1). An integrated platform of store‐and‐forward teledermatology consults within the electronic medical record (Epic, Madison, WI) was used with a secure smart phone application (Haiku or Canto; Epic). Team members utilized the Cisco WebEx virtual conference call system to conduct Health Insurance Portability and Accountability Act compliant discussions about patients. Clinical data were abstracted by a member of the dermatology consult service.
FIGURE 1.
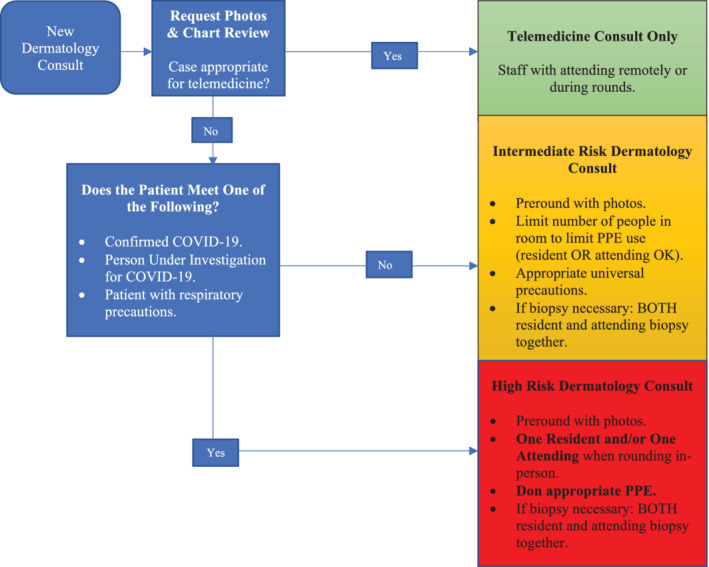
Algorithm for the use of store‐and‐forward teledermatology—algorithm developed particularly for COVID‐19 in which limiting in‐person patient encounters can decrease virus transmission
Sixteen patients (nine women and seven men) were evaluated using store‐and‐forward teledermatology services (Table 1). The most common consulting services were hematology and internal medicine (both 37.5%). In 43.8% of cases, the consulting service did not have an initial diagnosis for the patient. A median of 8 photographs (IQR 3‐17 photographs) were provided for each patient. Half of photographs were determined to be high quality, while half were moderate quality. At the consult date, nearly all patients had unknown COVID‐19 status (93.8%) and only 25% of patients had a negative final COVID‐19 diagnosis. Two physicians avoided unnecessary daily contact with 11 patients. Five of these patients ultimately required in‐person evaluation by dermatology team members, in which three punch biopsies and one shave biopsy were performed. In utilizing teledermatology, 20 pairs of gloves, 16 gowns, 10 N95 masks, and 4 surgical masks were conserved over the course of a single week.
TABLE 1.
Patients seen during teledermatology pilot study—descriptive characteristics of patients seen over the course of 1 week by the inpatient dermatology service at Ohio State University Wexner Medical Center
| # | Age (years) | Sex | Consulting service | Consulting service diagnosis | Dermatology initial diagnosis | Certainty of initial diagnosis | Dermatology final diagnosis | Number of photos Provided | Photo quality | Teletriage level of comfort | Patient seen in person? | Days until seen in person | Reason | Level of precautions at time of consult | COVID‐19 status at consult | Final COVID‐19 status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | Internal medicine | Shingles | Shingles | Moderate | ACD with Id reaction | 19 | High | Moderate | Yes | 2 | Unable to confirm diagnosis | Contact | Unknown, not tested | Unknown, not tested |
| 2 | 21 | F | Internal medicine | None | Morbilliform drug | High | Morbilliform drug | 6 | High | Moderate | Yes | 0 | Other ‐ assess for necrosis | Contact and airborne | Unknown, not tested | Negative |
| 3 | 74 | M | Oncology | None | Cutaneous metastases | Moderate | Cutaneous metastases | 7 | High | High | Yes | 0 | Essential condition warranting in person treatment | Universal | Unknown, not tested | Unknown, not tested |
| 4 | 45 | F | Internal medicine | SJS/TEN | Nutritional dermatitis | Moderate | Nutritional dermatitis, vasopressor‐induced skin necrosis | 35 | Moderate | High | No | N/A | N/A | Contact and airborne | Unknown, not tested | Negative |
| 5 | 64 | M | Hematology | None | Drug‐induced acneiform eruption | Somewhat | Drug‐induced acneiform eruption | 3 | High | Moderate | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 6 | 27 | F | Internal medicine | Eczema flare v. cellulitis | Eczema flare | High | Eczema flare | 17 | Moderate | High | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 7 | 62 | M | Neurosurgery | None | ACD with possible herpeticum and/or impetiginization | Moderate | ACD | 8 | Moderate | Moderate | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 8 | 60 | M | Internal medicine | Cellulitis | ACD | Moderate | ACD | 3 | High | High | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 9 | 58 | M | Hematology | None | Vasculitis, toxic erythema of chemotherapy | High | Drug‐induced purpura | 14 | Moderate | Moderate | Yes | 1 | Essential condition warranting in person treatment | Universal | Unknown, not tested | Unknown, not tested |
| 10 | 42 | F | Gynecology‐oncology | None | Allergic vs irritant contact dermatitis | High | Allergic vs irritant contact dermatitis | 20 | Moderate | High | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 11 | 29 | M | Hematology | Vasculitis | Ischemia 2/2 thromboembolism | Moderate | Ischemia due to thromboembolism | 3 | Moderate | Moderate | No | N/A | N/A | Universal | Unknown, not tested | Unknown, not tested |
| 12 | 47 | M | Internal medicine | None | HSV | Moderate | HSV | 1 | High | Moderate | No | N/A | N/A | Contact | Unknown, not tested | Unknown, not tested |
| 13 | 69 | F | Hematology | Leukemia cutis | Bite fibroma, NMSC | High | Bite fibroma, NMSC | 2 | High | High | No | N/A | N/A | Neutropenic, contact, and droplet | Negative | Negative |
| 14 | 49 | F | Hematology | Drug allergy | Toxic erythema of chemotherapy | Somewhat | Toxic erythema of chemotherapy | 16 | High | Moderate | No | N/A | N/A | Neutropenic | Unknown, not tested | Unknown, not tested |
| 15 | 56 | F | Hematology | Calciphylaxis | Calciphylaxis | Moderate | Thrombotic vasculopathy | 8 | Moderate | Moderate | Yes | 1 | Unable to confirm diagnosis | Universal | Unknown, not tested | Unknown, not tested |
| 16 | 81 | F | Oncology | Bullous pemphigoid flare | Bullous pemphigoid flare | High | Bullous pemphigoid flare | 10 | Moderate | Moderate | No | N/A | N/A | Contact and airborne | Unknown, not tested | Negative |
Abbreviations: ACD, allergic contact dermatitis; COVID‐19, coronavirus disease 2019; HSV, Herpes simplex virus; NMSC, non‐melanoma skin cancer; SJS/TEN, Stevens‐Johnson syndrome and toxic epidermal necrolysis.
Our findings demonstrate store‐and‐forward teledermatology can reduce unnecessary in‐person patient evaluation and management. Past reports of store‐and‐forward teledermatology use in clinic settings found as many as 71% of cases resulted in new diagnoses with treatment changes in 60% of patients. 1 In our study, 13 of 16 (81.3%) of electronic consultations resulted in new diagnoses, which informed treatment changes. Limiting in‐person interactions are essential to mitigating transmission of novel coronavirus SARS‐CoV‐2, which can persist on surfaces for 72 hours 2 and be transmitted by asymptomatic individuals. 3 In addition, PPE is increasingly scarce and expensive, prompting the Journal of the American Medical Association to publish an editorial soliciting creative ideas. 4 Our findings suggest that teledermatology may be used in inpatient settings during the COVID‐19 pandemic to conserve precious resources.
Limitations of our study include the small sample size, lack of a control group, and retrospective nature of the study. Our study also lacked metrics to evaluate the effect of inpatient teledermatology on quality of patient care and resident education. Nonetheless, we believe that our data suggest the need for greater investigation of this issue and validation of our results with larger studies under normal circumstances.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1. McAfee JL, Vij A, Warren CB. Store‐and‐forward teledermatology improves care and reduces dermatology referrals from walk‐in clinics: a retrospective descriptive study. J Am Acad Dermatol. 2020;82(2):499‐501. 10.1016/j.jaad.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 2. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;2019:577‐582. 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:2019‐2020. 10.1056/nejmc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment‐a call for ideas. JAMA. 2020;323:1911. 10.1001/jama.2020.4770. [DOI] [PubMed] [Google Scholar]


