Abstract
SARS‐CoV‐2 become pandemics and there is still a dearth of data about its the potentially among dermatological patients under biologics. We aimed to assess health literacy, disease knowledge, treatment dissatisfaction and biologics attitudes toward COVID‐19. We performed a cross‐sectional, questionnaire‐based survey on 98/105 consecutive dermatological patients treated with biologics—51 suffering from plaque psoriasis, 22 from atopic dermatitis, and 25 from hidradenitis suppurativa. An ad hoc, validated questionnaire has 44 items investigating the following domains: knowledge of COVID‐19 related to (a) epidemiology, (b) pathogenesis, (c) clinical symptoms, (d) preventive measures, and (e) attitudes. Patients data and questionnaires were collected. Despite only 8.1% thought that biologics may increase the risk of COVID‐19, 18.4% and 21.4% of the patients were evaluating the possibility to discontinue or modify the dosage of the current biologic therapy, respectively. Globally, male patients (P = .001) with higher scholarity level (P = .005) displayed higher knowledge of COVID‐19. Patients with lower DLQI (P = .006), longer disease duration (P = .051) and lower scholarity (P = .007) have thought to discontinue/modify autonomously their biologic therapy. At the multivariate logistic regression, only the knowledge of epidemiology and preventive measures resulted independent predictors of continuation vs discontinuation and modification vs no modification, respectively. Dermatologists should promote COVID‐19 knowledge to prevent biologics disruption.
Keywords: atopic dermatitis, biologics, COVID‐19, COVID‐19 questionnaire, hidradenitis suppurativa, psoriasis, SARS‐CoV‐2
1. INTRODUCTION
Since late December 2019 from Wuhan (Hubei province, People's Republic of China) a new Coronavirus, also known as SARS‐CoV2, has spread out in neighboring countries leading the Director‐General of the World Health Organization (WHO) to declare pandemics on March 11, 2020. 1 , 2 Rapidly, Italy has become red‐zone with the highest rate of COVID‐19 confirmed, hospitalized and deceased patients in Europe; thus to handle this massive health emergency several medical departments were reconverted in COVID‐19‐dedicated or partially dedicated units, dermatology had promoted telemedicine and maintained face‐to‐face visits only for urgent patients (ie, melanoma surgery) and chronic patients under certain systemic drugs (ie, biologics and other immunosuppressants). 3
COVID‐19 pandemic has forced everyone to use personal protective equipment (PPE), such as goggles, N95 masks, double‐layers gloves, and face‐shields, and to follow methodically sanitization protocols. 2 Hence, health care workers due to too scrupulous and continuous hand‐washing and use of preventive measures and protective equipment could develop hand eczema and related skin disorders. 4 Lan and colleagues recruited a sample of 542 health care and in 97% of them they found a dermatological disorder related to the personal protective equipment (PPE) and to the preventive measures, mainly affecting the nasal bridge, the hands, the cheeks and the forehead, with dryness and desquamation being the most commonly reported symptoms/signs. 5 However, mainly occupational aspects have been investigated so far.
To the best of our knowledge, there is a dearth of data concerning the COVID‐19 perceptions of dermatological patients under biologics, a therapy traditionally associated to an increased risk of infections. 6 , 7 , 8 , 9 This aspect is of particular interest since it may affect the patients' compliance leading to treatment discontinuation or autonomous modifications. 10 Although biologics have revolutionized the management of chronic dermatological disorders, their interplay between disease, disease activity, and its pharmacological treatment is complex and multifaceted, and sometimes drug‐related side effects may occur (ie, airway infections). Side effects are also capable to detriment dermatologist‐patients relationship leading to a decreased compliance. 11 Furthermore, also inside the dermatological field the attitude towards biologics are discordant 12 , 13 due to the dearth of available data.
In these historical and scientific context of uncertainty, in which hospitals are overwhelmed by COVID‐19 emergency and at the same time are struggled also by the normal routine (acute patients and chronic ones), we decided to perform a study to assess how COVID‐19 impacts patients under biologics to optimize our daily approach.
2. MATERIAL AND METHODS
2.1. Ethical clearance
The protocol study of the present investigation was in‐depth reviewed, respected the ethical principles of seventh Helsinki Declaration and received full ethical clearance by the involved Institutions. All patients signed a written consent form.
2.2. Patients selection: inclusion and exclusion criteria
This cross‐sectional, questionnaire‐based survey was performed in February 10, 2020, before the declaration of pandemics, in three primary referral dermatological centers, IRCCS Galeazzi Orthopedic Istitute, IRCCS San Donato, both in Milan, and IRCCS San Gallicano in Rome. All the clinical evaluations were coherent with Italian Society of Dermatology, Venereology and Sexual Transmitted Diseases (SIDEMAST) recommendations during COVID‐19 pandemics (www.sidemast.org/blog/coronavirus). Patients scheduled for these days were consecutively enrolled if they met the eligible criteria.
Patients were enrolled in the present study if meeting the following inclusion criteria: (a) aged ≥18 years, (b) diagnosis of plaque psoriasis, atopic dermatitis or hidradenitis suppurativa performed by two independent board‐certified dermatologists lasting more than 5 years ago, (c) with a severity.
in psoriatic patients: Psoriasis Area Severity Index (PASI) 14 ≥10 and or Disease Activity index for PSoriatic Arthritis” (DAPSA) 15 > 14 before starting the systemic treatment and a stable disease (Delta PASI or Delta DAPSA in two consecutive controls <10%) at the study baseline;
in atopic dermatitis patients with Eczema Area and Severity Index (EASI) 16 >22 before starting the systemic treatment and a stable disease (Delta EASI in two consecutive controls <10%) at the study baseline;
in HS patients with Hurley III 17 and International Hidradenitis Suppurativa Severity Score System (IHS4) 18 >10 before starting the systemic treatment and a stable disease (Delta IHS4 in two consecutive controls <10%) at the study baseline,
(d) under biologics treatment for >1 year.
Patients were excluded if: (a) history or actual diagnosis of psychiatric disease, (b) diagnosed degenerative neurological disease (acquired or congenital), (c) previous chemotherapy, (d) brain tumor, (e) drug addictions, (f) <1 year of treatment with biologics, (g) <5 years disease duration.
Remarkably, in these departments patients undergoing a biological therapy were affecting only by psoriasis (PsO), or atopic dermatitis (AD) or hidradenitis suppurativa (HS).
2.3. Dermatological assessment
After verifying medical history and demographics already recorded in the database, two board‐certified, independent dermatologists clinically assessed the enrolled patients collecting the appropriate severity scores in compliance with the Italian guidelines. 19 , 20 , 21 , 22 , 23
AD patients were evaluated using Dermatologic Quality of Life Score (DLQI) 23 , 24 and Eczema Area and Severity Index (EASI). PsO patients were evaluated using DLQI, PASI and DAPSA (if psoriatic arthritis was co‐diagnosed), whilst HS patients underwent DLQI, Hurley score, IHS4 and Autoinflammatory Disease Damage Index (ADDI). 25
2.4. Questionnaire development
A validated questionnaire consisting of 44 items was administered to a cohort of patients with dermatological disorders 26 (Supplementary material 1). The questionnaire was comprised of five sections: the first assessed the risk perception about the likelihood of becoming infected by the SARS‐CoV2 and negative attitudes towards the pharmacological treatment, the second explored the knowledge regarding the virus, the third the knowledge concerning the clinical symptoms and manifestations, the fourth preventive measures that can be implemented against COVID‐19 and, finally, the fifth the risk perception.
2.5. Statistical analysis
Before commencing any statistical analyses, data were visually inspected for capturing potential outliers. Descriptive statistics was performed, by expressing values as means ± SDs. Scores were also assessed in terms of kurtosis and skewness. Regression analyses were carried out to shed light on the determinants of the knowledge score. All statistical analyses were carried out by means of the commercial software “Statistical Package for Social Sciences” (SPSS version 24 for Windows, IBM Corporation, Armonk, New York). Graphs were generated by means of the commercial software MedCalc Statistical Software (version 18.11.3, MedCalc Software bvba, Ostend, Belgium, 2019). All figures with P‐values less than or equal to .05 were considered statistically significant.
3. RESULTS
3.1. Clinical and demographic data
We interviewed 105 consecutive dermatological patients under biologics and 98 (93.3%) were enrolled, 51 (52.0%) suffering from plaque psoriasis, 22 (22.4%) from atopic dermatitis, and 25 (25.5%) from hidradenitis suppurativa. Among psoriatic patients only 27/51 (52.9%) have also psoriatic arthritis. The mean age in the enrolled patients was 44.36 ± 8.45 years (median 43 years) (PsO: 46.35 ± 9.02, AD: 40 ± 6.90, HS: 44.12 ± 7.18) with a mean disease duration of 17.77 ± 7.19 years (median 17 years) (PsO: 17.35 ± 7.07, AD: 21.55 ± 8.07, HS: 15.28 ± 5.28). Median DLQI was 12 (12.3 ± 2.8) (PsO: 10.86 ± 2.47, AD: 13.68 ± 2.38, HS: 14.16 ± 2.17). PASI and DAPSA among psoriatic patients were 2.9 ± 2.2 (median 3) and 6.2 ± 3.7 (median 6). In HS patients IHS4 and ADDI were 7.8 ± 3.4 (median 8) and 2.7 ± 0.8 (median 3) respectively. In AD patients the EASI was 7.8 ± 2.6 (median 8). From a therapeutic point of view, the enrolled patients underwent Adalimumab (n = 36, 36.7%), Dupilumab (n = 22, 22.4%), Etanercept (n = 13, 13.3%), Ustekinumab (n = 10, 10.2%), Ixekizumab (n = 8, 8.2%), Secukinumab (n = 7, 7.1%) and Certolizumab 2 (2.0%). Further details are shown in Table 1.
TABLE 1.
Main characteristics of the recruited sample
| Variable | Value |
|---|---|
| Sociodemographic parameters | |
|
Age Gender Male Female Family history Scholarity Primary school Middle school High school University PhD/master |
44.36 ± 8.45 (43) 51 (52.0%) 47 (48.0%) 38 (38.8%) 3 (3.1%) 14 (14.3%) 35 (35.7%) 35 (35.7%) 11 (11.2%) |
| Disease | |
|
Plaque psoriasis Hidradenitis suppurativa Atopic dermatitis |
51 (52.0%) 25 (25.5%) 22 (22.4%) |
| Disease severity | |
|
Disease duration DLQI |
17.77 ± 7.19 (17) 12.3 ± 2.8 (12) |
| Psoriasis | |
|
PASI DAPSA |
2.9 ± 2.2 (3) 6.2 ± 3.7 (6) |
| Hidradenitis suppurativa | |
|
IHS4 ADDI |
7.8 ± 3.4 (8) 2.7 ± 0.8 (3) |
| Atopic dermatitis | |
| EASI | 7.8 ± 2.6 (8) |
| Biologic therapies | |
|
Adalimumab Dupilumab Etanercept Ustekinumab Ixekizumab Secukinumab Certolizumab |
36 (36.7%) 22 (22.4%) 13 (13.3%) 10 (10.2%) 8 (8.2%) 7 (7.1%) 2 (2.0%) |
Abbreviations: ADDI, Autoinflammatory Disease Damage Index; DAPSA, Disease Activity Index for PSoriatic Arthritis; DLQI, Dermatologic Life Quality Score; EASI, Eczema Area and Severity Index; IHS4, International Hidradenitis Suppurativa Severity Score System; PASI, Psoriasis Area Severity Index.
3.2. COVID‐19 risk perceptions and relative attitudes
Scores for each domain and for the overall questionnaire are reported in Table 2. Noteworthy, no differences among the disease groups could be found, so the entire sample of dermatological patients was analyzed in an aggregated manner (Figure 1). SARS‐CoV2 infection worried half of the interviewed patients, in particular 25 (25.6%) were really worried, 24 (24.5%) moderately worried, 29(29.6%) a little worried and 20 (20.4%) not worried at all.
TABLE 2.
Scores of each domain of the questionnaire utilized in the present study
| Questionnaire domain | Value | Range | ||
|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | |
| COVID‐19 related epidemiology | 39.22 | 5.00 | 27 | 57 |
| COVID‐19 related pathogenesis | 28.64 | 5.57 | 0 | 42 |
| COVID‐19 related clincal symptoms | 25.40 | 5.43 | 13 | 37 |
| COVID‐19 related prevention | 12.12 | 2.79 | 6 | 18 |
| COVID‐19 related attitudes | 12.39 | 2.29 | 9 | 18 |
| Total COVID‐19 related knowledge and attitudes score | 117.78 | 9.41 | 71 | 136 |
FIGURE 1.
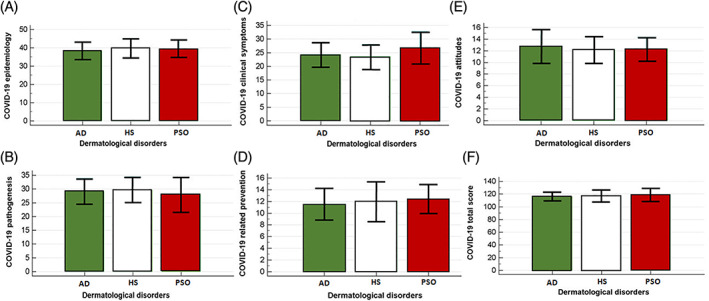
Knowledge score of COVID‐19 related risk perceptions and epidemiology, A; pathogenesis, B; clinical symptoms, C; preventive measures, D; attitudes, E; and overall score, F; stratified according to the dermatological disorders of the patients recruited (atopic dermatitis. Hidradenitis suppurativa and plaque psoriasis)
Remarkably, 28 (28.6%) patients perceived that their chronic dermatological disease expose them to a moderate‐to‐severe risk to contract SARS‐CoV2, whereas 17.3% and 54.1% regard it as low or null. Despite only 8.1% thought that biologics expose them to a moderate to severe risk to contract SARS‐CoV2, 18.4% and 21.4% of the whole patients declared that they have assessed the possibility to discontinue or modify the dosage of the current biologic therapy, respectively.
3.3. Clinical variables influencing COVID‐19 questionnaire domains
At the multivariate regression analysis, knowledge regarding the virus epidemiology was found to correlate with male gender (coefficient regression 2.59, P = .01) and scholarity level (coefficient regression 1.80, P = .0003).
Knowledge of COVID‐19 related pathogenesis was associated with DLQI (coefficient regression 0.61, P = .0061) and inversely with scholarity level (coefficient regression −1.03, P = .0620, significantly borderline).
Knowledge concerning clinical symptoms inversely correlated with DLQI (coefficient regression −0.80, P = .0001), and directly with scholarity level (coefficient regression 1.40, P = .0058).
Knowledge concerning prevention inversely correlated with DLQI (coefficient regression −0.33, P = 0.0019) and positively with scholarity level (coefficient regression 1.00, P = .0002).
COVID‐19 related attitudes (drug continuation vs modification/discontinuation) directly correlated with DLQI (coefficient regression 0.24, P = .0059), disease duration (coefficient regression 0.07, P = .0513, statistically borderline) and inversely with scholarity (coefficient regression −0.59, P = .0077).
Globally male patients (coefficient regression 6.97, P = .0003) with higher scholarity level (coefficient regression 2.57, P = .0049) displayed higher knowledge of COVID‐19. Further details are reported in Table 3.
TABLE 3.
Multivariate regression analyses for the scores of each domain and the overall score of the COVID‐19 related knowledge and attitudes questionnaire utilized in the present study
| Independent variables | Coefficient | SE | t | P | r partial | r semipartial |
|---|---|---|---|---|---|---|
| COVID‐19 related knowledge concerning epidemiology | ||||||
| (Constant) | 36.78 | |||||
| Age | 0.04 | 0.07 | 0.52 | .6069 | .05 | .05 |
| Male gender | 2.59 | 0.98 | 2.63 | .0100 | .27 | .25 |
| Disease | −0.70 | 0.75 | −0.93 | .3531 | −.10 | .09 |
| Disease duration | −0.12 | 0.08 | −1.55 | .1238 | −.16 | .15 |
| Family history | −0.59 | 0.98 | −0.60 | .5523 | −.06 | .06 |
| DLQI | −0.21 | 0.19 | −1.10 | .2740 | −.12 | .10 |
| Scholarity | 1.80 | 0.48 | 3.78 | .0003 | .37 | .35 |
| COVID‐19 related pathogenesis | ||||||
| (Constant) | 24.61 | |||||
| Age | −0.02 | 0.08 | −0.27 | .7919 | −.03 | .03 |
| Male gender | 1.81 | 1.13 | 1.60 | .1129 | .17 | .15 |
| Disease | 0.17 | 0.86 | 0.20 | .8458 | .02 | .02 |
| Disease duration | −0.03 | 0.09 | −0.30 | .7617 | −.03 | .03 |
| Family history | 0.27 | 1.13 | 0.24 | .8145 | .02 | .02 |
| DLQI | 0.61 | 0.22 | 2.81 | .0061 | .28 | .27 |
| Scholarity | −1.03 | 0.55 | −1.89 | .0620 | −.20 | .18 |
| COVID‐19 related knowledge concerning clinical symptoms | ||||||
| (Constant) | 30.82 | |||||
| Age | −0.01 | 0.07 | −0.11 | .9155 | −.01 | .01 |
| Male gender | 1.69 | 1.03 | 1.65 | .1022 | .17 | .15 |
| Disease | −0.12 | 0.78 | −0.15 | .8786 | −.02 | .01 |
| Disease duration | −0.02 | 0.08 | −0.25 | .8061 | −.03 | .02 |
| Family history | −0.46 | 1.03 | −0.45 | .6518 | −.05 | .04 |
| DLQI | −0.80 | 0.20 | −4.06 | .0001 | −.39 | .36 |
| Scholarity | 1.40 | 0.50 | 2.83 | .0058 | .29 | .25 |
| COVID‐19 related knowledge of preventive measures | ||||||
| (Constant) | 13.58 | |||||
| Age | 0.003 | 0.04 | 0.10 | .9230 | .01 | .01 |
| Male gender | 0.72 | 0.53 | 1.34 | .1835 | .14 | .12 |
| Disease | −0.32 | 0.41 | −0.80 | .4289 | −.08 | .07 |
| Disease duration | −0.02 | 0.04 | −0.55 | .5850 | −.06 | .05 |
| Family history | −0.42 | 0.53 | −0.79 | .4317 | −.08 | .07 |
| DLQI | −0.33 | 0.10 | −3.19 | .0019 | −.32 | .29 |
| Scholarity | 1.00 | 0.26 | 3.87 | .0002 | .38 | .35 |
| COVID‐19 related attitudes | ||||||
| (Constant) | 8.7714 | |||||
| Age | 0.01 | 0.03 | 0.22 | .8288 | .02 | .02 |
| Male gender | 0.16 | 0.45 | 0.36 | .7217 | .04 | .03 |
| Disease | 0.35 | 0.34 | 1.02 | .3090 | .10 | .10 |
| Disease duration | 0.072 | 0.04 | 1.98 | .0513 | .20 | .18 |
| Family history | 0.33 | 0.45 | 0.72 | .4718 | .08 | .07 |
| DLQI | 0.24 | 0.09 | 2.82 | .0059 | .29 | .26 |
| Scholarity | −0.59 | 0.22 | −2.73 | .0077 | −.28 | .25 |
| COVID‐19 related total knowledge and attitudes score | ||||||
| (Constant) | 114.56 | |||||
| Age | 0.02 | 0.13 | 0.14 | .8930 | .01 | .01 |
| Male gender | 6.97 | 1.84 | 3.79 | .0003 | .37 | .35 |
| Disease | −0.62 | 1.40 | −0.44 | .6578 | −.05 | .04 |
| Disease duration | −0.12 | 0.15 | −0.83 | .4085 | −.09 | .08 |
| Family history | −0.88 | 1.84 | −0.48 | .6335 | −.05 | .04 |
| DLQI | −0.48 | 0.35 | −1.35 | .1791 | −.14 | .13 |
| Scholarity | 2.57 | 0.89 | 2.89 | .0049 | .29 | .27 |
Abbreviation: DLQI, Dermatologic Life Quality Index; SE, standard error.
3.4. Therapy attitudes and COVID‐19 questionnaire
Stratifying according to continuation vs discontinuation and no modification vs modification in drug dose/schedule, statistically significant differences in terms of knowledge of COVID‐19 related epidemiology, pathogenesis, clinical symptoms and preventive measures (all, P‐value <.001) were found. Noteworthy, scores were higher in the continuation/no modification group, except for knowledge of COVID‐19 related pathogenesis, for which higher scores were reported in the discontinuation/modification group. No differences could be found in terms of age, gender distribution, scholarity level, family history, disease type, disease duration and DLQI score. More details are shown in Table 4.
TABLE 4.
Univariate analysis showing statistically significant differences between continuation/no modification and discontinuation/modification groups
| Domain | Continuation | Discontinuation | P‐value | No modification | Modification | P‐value |
|---|---|---|---|---|---|---|
| Epidemiology | 40.45 ± 4.31 | 33.78 ± 4.07 | < .001 | 40.60 ± 4.24 | 34.19 ± 4.24 | < .001 |
| Pathogenesis | 27.86 ± 4.33 | 32.11 ± 8.64 | < .001 | 27.57 ± 4.24 | 32.57 ± 7.85 | < .001 |
| Clinical symptoms | 26.70 ± 4.94 | 19.61 ± 3.46 | < .001 | 26.97 ± 4.64 | 19.62 ± 4.08 | < .001 |
| Preventive measures | 12.74 ± 2.66 | 9.39 ± 1.29 | < .001 | 12.97 ± 2.45 | 9.00 ± 1.34 | < .001 |
At the multivariate logistic regression, only knowledge of COVID‐19 ‐related epidemiology (OR 0.81 [95%CI 0.67‐0.98], P = .0334) and of COVID‐19‐related preventive measures (OR 0.54 [95%CI 0.34‐0.5], P = .0075) resulted independent predictors (more precisely, protective factors) of continuation vs discontinuation and modification vs no modification, respectively (Table 5).
TABLE 5.
Multivariate logistic regression analyses shedding light on the determinants of continuation vs discontinuation and modification vs no modification of biologic therapies in the considered sample of dermatological patients
| Variable | Coefficient | SE | Wald | P‐value | OR | 95%CI |
|---|---|---|---|---|---|---|
| Continuation vs discontinuation | ||||||
| Constant | 10.56 | 3.61 | 8.54 | .0035 | ||
| Epidemiology | −0.21 | 0.10 | 4.53 | .0334 | 0.81 | 0.67–0.98 |
| Pathogenesis | 0.03 | 0.05 | 0.31 | .5796 | 1.03 | 0.93‐1.14 |
| Clinical symptoms | −0.10 | 0.10 | 1.41 | .2358 | 0.90 | 0.76‐1.07 |
| Prevention | −0.26 | 0.19 | 1.93 | .1644 | 0.77 | 0.53‐1.11 |
| Modification vs no modification | ||||||
| Constant | 11.96 | 3.92 | 9.30 | .0023 | ||
| Epidemiology | −0.14 | 0.10 | 2.29 | .1302 | 0.87 | 0.72‐1.04 |
| Pathogenesis | 0.03 | 0.05 | 0.41 | .5212 | 1.03 | 0.93–1.14 |
| Clinical symptoms | −0.10 | 0.10 | 1.28 | .2580 | 0.90 | 0.76‐1.08 |
| Prevention | −0.62 | 0.23 | 7.14 | .0075 | 0.54 | 0.34‐0.85 |
4. DISCUSSION
During COVID‐19 pandemics ~40% of dermatological patients under biologics have thought to autonomously modify or even discontinue their therapy.
SARS‐CoV2 displayed a special tropism for respiratory epithelium, thus it may cause respiratory symptoms of different severity spacing from mild cough to death in 7.2% of the cases in Italy. 27 , 28 Since COVID‐19 pathogenesis involved mainly respiratory airways, patients with respiratory comorbidities might have higher risk, but at the moment no data are present to confirm it. 29 In literature, both psoriasis, atopic dermatitis and hidradenitis suppurativa displayed an higher risk of respiratory comorbidities; in accord with this evidence ~30% of the interviewed patients thought that their dermatological disease could increase the SARS‐CoV2 infection risk.
Psoriatic patients displayed a baseline airway inflammation, 30 , 31 that may lead to the epidemiologically proven increased risk of asthma, and chronic obstructive pulmonary disease (COPD). 32 AD theory of “atopic march” gives the pathogenetic rationale to the increased asthma risk found in atopic patients. 33 Then, HS and PsO patients, there is an high prevalence of smokers and in both disease smoking increase the severity and flares. 34 , 35 Interestingly, Lippi and colleagues found that active smoking is not correlated with COVID‐19 severity. 36
Beside the direct effects of the dermatological disease, the impact of biologics on SARS‐CoV2 infection risk were regarded as negligible in our patients, in fact only 1 in 10 interviewed patients thought that biologics may increase their risk to contract COVID‐19. Despite only 8.1% thought that biologics expose them to a moderate to severe risk to contract COVID‐19, 18.4% and 21.4% of the whole patients declared that they have assessed the possibility to discontinue or modify the dosage of the current biologic therapy, respectively.
Biologics have revolutionized the treatment and management of chronic dermatological disorders, but they also have increased the rate of airway infections, especially for psoriasis and hidradenitis suppurativa. 12 , 13 , 36 , 37 Conversely, in a recent meta‐analysis Zayed and colleagues did not find an increased risk of airway infections in AD patients with asthma undergoing dupilumab. 38 No data are still present about the SARS‐CoV2 increased risk of infection in patients undergoing biologics, but the present literature may justify the therapeutic doubts occurred in ~40% of our patients. Otherwise, transplanted patients undergoing immunosuppressants, communed by a dysfunctional immune system seem to not have an increased risk to contract Coronavirus. 39 , 40
Our data suggest that the knowledge about COVID‐19 may influence the therapy discontinuation, in fact COVID‐19‐related epidemiology information was a protective factor for biologics discontinuation, while the COVID‐19‐related information on preventive measures was a protective factor for biologics dosage modification. Furthermore, scholarity level positive correlates with both prevention and epidemiology domains, but inversely correlates with pathogenesis domain. To further confirm, COVID‐19 related attitudes to modify/discontinue biologics directly correlated with DLQI, disease duration and inversely with scholarity. In literature both scholarity and educational interventions are capable to increase drug adherence and compliance. 41 , 42 , 43 Recently, guidelines and vademecum for patients and dermatologists were produced by the Italian Dermatologists Society (SIDEMAST), however the dermatological world is still discordant on use of biologics during COVID‐19 pandemics. 12 , 13 Furthermore, also during the overwhelming emergency, 44 , 45 , 46 dermatologists should dedicate time to discuss COVID‐19 insights with patients undergoing biologics in order to prevent their loss of compliance.
However, the present study is not without any limitation. The major shortcoming is represented by the relatively small sample size employed. Furthermore, the knowledge was limited to pre‐pandemic period. It would be interesting to evaluate knowledge of dermatological patients undergoing biologics also in postpandemic period.
5. CONCLUSION
The knowledge of COVID‐19 has a paramount importance in dermatological patients undergoing biologics and dermatologists should promote it. Therapy continuation during COVID‐19 emergency seems to strictly depend on the quality of information that patients acquire. Discontinuing or modifying biologic therapy expose patients to the risk of losing response to a drug previously useful.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Supplementary material
Bragazzi NL, Riccò M, Pacifico A, et al. COVID‐19 knowledge prevents biologics discontinuation: Data from an Italian multicenter survey during RED‐ZONE declaration. Dermatologic Therapy. 2020;33:e13508. 10.1111/dth.13508
Paolo Pigatto and Giovanni Damiani contributed equally to this work.
REFERENCES
- 1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020;94:44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacifico A, Ardigò M, Frascione P, Damiani G, Morrone A. Phototherapeutic approach to dermatological patients during the 2019 coronavirus pandemic: real‐life data from the Italian red zone. Br J Dermatol. 2020. 10.1111/(ISSN)1365-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elston DM. Occupational skin disease among healthcare workers during the coronavirus (COVID‐19) epidemic. J Am Acad Dermatol. 2020;82:1085‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lan J, Song Z, Miao X, et al. Skin damage and the risk of infection among healthcare workers managing coronavirus disease‐2019. J Am Acad Dermatol. 2020;82:1215‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS‐CoV‐2 infection and hospitalization, but not ICU admission and death: real‐life data from a large cohort during RED‐ZONE declaration. Dermatol Ther. 2020;e13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Andersen KM, Chang HY, Curtis JR, Alexander GC. Comparative risk of serious infections among real‐world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis. 2020;79(2):285‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellis A, Khanna U, Galadari A, Fernandez AP. Conversion to positive latent tuberculosis infection status is low in Hidradenitis Suppurativa patients taking biologic medications. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 9. Thaçi D, L Simpson E, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate‐to‐severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019;94(2):266‐275. [DOI] [PubMed] [Google Scholar]
- 10. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Schoot LS, van den Reek JMPA, Groenewoud JMM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side‐effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol. 2019;33(10):1913‐1920. [DOI] [PubMed] [Google Scholar]
- 12. Conforti C, Giuffrida R, Dianzani C, Di Meo N, Zalaudek I. COVID‐19 and psoriasis: is it time to limit treatment with immunosuppressants? A call for action. Dermatol Ther. 2020;e13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol. 2020;82:1217‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157(4):238‐244. [DOI] [PubMed] [Google Scholar]
- 15. Nell‐Duxneuner VP, Stamm TA, Machold KP, Pflugbeil S, Aletaha D, Smolen JS. Evaluation of the appropriateness of composite disease activity measures for assessment of psoriatic arthritis. Ann Rheum Dis. 2010;69(3):546‐549. [DOI] [PubMed] [Google Scholar]
- 16. Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 17. Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus. Surgical approach. In: Roenigk R, Roenigk H, eds. Dermatologic Surgery, Principles and Practice. New York, New York: Marcel Dekker; 1989. [Google Scholar]
- 18. Zouboulis CC, Tzellos T, Kyrgidis A, et al. Development and validation of the international Hidradenitis Suppurativa severity score system (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401‐1409. [DOI] [PubMed] [Google Scholar]
- 19. Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774‐790. [DOI] [PubMed] [Google Scholar]
- 20. Damiani G, Calzavara‐Pinton P, Stingeni L, et al. Italian guidelines for therapy of atopic dermatitis‐adapted from consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis). Dermatol Ther. 2019;32(6):e13121. [DOI] [PubMed] [Google Scholar]
- 21. Megna M, Bettoli V, Chimenti S, et al. Hidradenitis suppurativa: guidelines of the Italian Society of Dermatology and Venereology (SIDeMaST) for the use of anti‐TNF‐α agents. G Ital Dermatol Venereol. 2015;150(6):731‐739. [PubMed] [Google Scholar]
- 22. Stingeni L, Bianchi L, Hansel K, et al. Italian guidelines in patch testing ‐ adapted from the European Society of Contact Dermatitis (ESCD). G Ital Dermatol Venereol. 2019;154(3):227‐253. [DOI] [PubMed] [Google Scholar]
- 23. Cazzaniga S, Naldi L, Damiani G, et al. Validation of a visual‐aided questionnaire for the self‐assessment of hidradenitits suppurativa. J Eur Acad Dermatol Venereol. 2018;32(11):1993‐1998. [DOI] [PubMed] [Google Scholar]
- 24. Finlay AY, Khan GK. Dermatology life quality index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210‐216. [DOI] [PubMed] [Google Scholar]
- 25. Damiani G, Della Valle V, Iannone M, Dini V, Marzano AV. Autoinflammatory disease damage index (ADDI): a possible newborn also in hidradenitis suppurativa daily practice. Ann Rheum Dis. 2017;76(8):e25. [DOI] [PubMed] [Google Scholar]
- 26. Riccò M, Ferraro P, Gualerzi G, Ranzieri S. Point‐of‐Care diagnostic of SARS‐CoV‐2: knowledge, attitudes, and beliefs (KAP) of medical workforce in Italy. Acta Biomed. 2020;91(2). 10.23750/abm.v91i2.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarzi‐Puttini P, Giorgi V, Sirotti S, et al. COVID‐19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020.38(2):337–342. [PubMed] [Google Scholar]
- 28. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020. 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 29. Wang Zhongliang, Yang Bohan, Li Qianwen, Wen Lu, Zhang Ruiguang. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clinical Infectious Diseases. 2020; 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Damiani G, Radaeli A, Olivini A, Calvara‐Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. 2016;175(4):797‐799. [DOI] [PubMed] [Google Scholar]
- 31. Malerba M, Damiani G, Radaeli A, Ragnoli B, Olivini A, Calzavara‐Pinton PG. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br J Dermatol. 2015;173(6):1544‐1545. [DOI] [PubMed] [Google Scholar]
- 32. Santus P, Rizzi M, Radovanovic D, et al. Psoriasis and respiratory comorbidities: the added value of fraction of exhaled nitric oxide as a new method to detect, evaluate, and monitor psoriatic systemic involvement and therapeutic efficacy. Biomed Res Int. 2018;2018:3140682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aw M, Penn J, Gauvreau GM, Lima H, Sehmi R. Atopic March: Collegium Internationale Allergologicum Update 2020. Int Arch Allergy Immunol. 2020;181(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 34. Naldi L. Psoriasis and smoking: links and risks. Psoriasis (Auckl). 2016;6:65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Acharya P, Mathur M. Hidradenitis suppurativa and smoking: a systematic review and meta‐analysis. J Am Acad Dermatol. 2020;82(4):1006‐1011. [DOI] [PubMed] [Google Scholar]
- 36. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med. 2020;75:107‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375(5):422‐434. [DOI] [PubMed] [Google Scholar]
- 38. Zayed Y, Kheiri B, Banifadel M, et al. Dupilumab safety and efficacy in uncontrolled asthma: a systematic review and meta‐analysis of randomized clinical trials. J Asthma. 2018;2018:1‐10. [DOI] [PubMed] [Google Scholar]
- 39. Gori A, Dondossola D, Antonelli B, et al. Coronavirus disease 2019 and transplantation: a view from the inside. Am J Transpl. 2020. 10.1111/ajt.15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020. 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Qiu Y, Zhao F, Zhang F. Poor medication adherence in patients with psoriasis and a successful intervention. J Dermatolog Treat. 2019;30(6):525‐528. [DOI] [PubMed] [Google Scholar]
- 42. Hawkins SD, Barilla S, Feldman SR. Web app based patient education in psoriasis ‐ a randomized controlled trial. Dermatol Online J. 2017;23(4):13030/qt26d525z5. [PubMed] [Google Scholar]
- 43. Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74(6):1057‐1065.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenbaum L. Facing Covid‐19 in Italy ‐ ethics, logistics, and therapeutics on the Epidemic's front line. N Engl J Med. 2020. 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 45. Radi G, Diotallevi F, Campanati A, Offidani A. Global coronavirus pandemic (2019‐nCOV): implication for an Italian medium size dermatological clinic of a ii level hospital. J Eur Acad Dermatol Venereol. 2020. 10.1111/jdv.16386. [DOI] [PubMed] [Google Scholar]
- 46. Lazzerini M, Putoto G. COVID‐19 in Italy: momentous decisions and many uncertainties. Lancet Glob Health. 2020;8(5):e641–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


