Abstract
Although immunosuppressed patients may be more prone to SARS‐CoV‐2 infection with atypical presentation, long‐term immunosuppression therapy may provide some sort of protection for severe clinical complications of COVID‐19. The interaction between immunosuppression and new antiviral drugs in the treatment of transplanted patients contracting COVID‐19 has not yet been fully investigated. Moreover, data regarding the optimal management of these patients are still very limited. We report a case of the successful recovery from severe COVID‐19 of a kidney‐transplanted patient treated with hydroxychloroquine, lopinavir/ritonavir, steroid, and tocilizumab.
Keywords: coronavirus, COVID‐19, immunosuppression, kidney transplantation, pandemic, tocilizumab
Abbreviations
- ARDS
acute respiratory distress syndrome
- CNIs
calcineurin inhibitors
- COVID‐19
coronavirus disease 2019
- CSA
cyclosporine
- EVL
everolimus
- GGO
ground‐glass opacification
- ICU
intensive care unit
- NIV
non‐invasive ventilation
- PCT
procalcitonin
- PEEP
positive end‐expiratory pressure
- RCP
reactive C protein
- RT‐PCR
real‐time reverse transcription‐polymerase chain reaction
- sCR
serum creatinine
1. INTRODUCTION
Our understanding of COVID‐19 epidemiology and its clinical presentation is growing fast, yet few reports of COVID‐19 among transplanted recipients have been published so far. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 The risk of immunocompromised patients developing atypical severe COVID‐19 is still under debate. 9
The severity of clinical manifestations and lung injury histology would seem especially to implicate over activation of the T‐cell immune response. 10 Long‐term immunosuppression with calcineurin inhibitors (CNIs) is known to affect T‐cell proliferation and maturation, with consequent anti‐inflammatory effects. 11 However, the question of whether long‐term immunosuppressive therapy could shorten or mitigate the clinical course of COVID‐19 in transplanted patients still awaits a definitive answer. Reports on the clinical course of transplant recipients treated with tocilizumab are scarce, and currently, only a few cases have been reported. 12
We present a case of successful recovery from severe COVID‐19 of a kidney‐transplanted patient treated according to our local protocol.
2. CASE REPORT
A 51‐year‐old woman underwent kidney transplantation (KT) in July 2017 for end‐stage glomerulonephritis.
Previous medical history included Pneumocystis jirovecii pneumonia and cytomegalovirus infection 1 year after KT, arterial hypertension, hypothyroidism, and recurrent urinary tract infection (UTI).
On March 10, 2020 (subsequently considered D0), she was admitted to the emergency room (ER) with fever (38.4°C), malaise, history of strangury, dry cough, and chest pain. Although the patient reported no clear contact with confirmed or suspected cases of COVID‐19, as the clinical presentation was consistent with COVID‐19 pneumonia, she underwent nasopharyngeal swab specimen and chest x‐ray.
Real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) assay for SARS‐CoV‐2 was positive.
Her current immunosuppressive therapy consisted of cyclosporine (CSA) 35 mg BID, everolimus (EVL) 1 mg BID, and prednisone 5 mg once a day. She was also being treated with angiotensin receptor 1 blocker, levothyroxine, and fosfomycin as UTI prophylaxis. She had never been on hemodialysis, and her most recent serum creatinine (sCr) was 1.36 mg/dL. CSA level was in the range of 100‐150 μg/L.
On admittance to ER, the patient's physical examination was unremarkable. Blood pressure was 140/85 mm Hg, pulse 100 beats per minute, and oxygen saturation was 96% in ambient air. Initial laboratory tests revealed sCr 2.4 mg/dL, procalcitonin (PCT) 0.28 ng/mL, reactive C protein (RCP) 7.5 mg/dL, and D‐dimer 0.67 μg/mL. A chest CT scan showed a single minor subpleural ground‐glass opacification (GGO) in the lower lobe of the right lung (Figure 1A). The laboratory tests and main events are summarized in Figure 2.
FIGURE 1.

High‐resolution CT images before and after treatment. A, The CT shows single minor subpleural ground‐glass opacification (GGO) in the lower lobe of the right lung (D0). B, the imaging confirmed disease progression to the left lung (D8). C, CT shows bilateral, multiple, and subpleural GGO and consolidation, and thickening of intralobular septa (crazy‐paving sign) (D14). D, TC shows interstitial fibrosis (D25)
FIGURE 2.
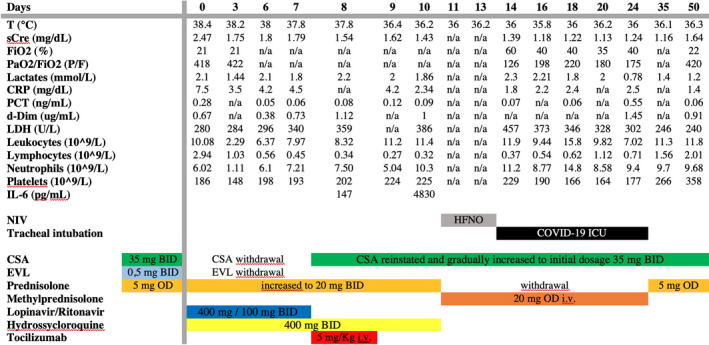
Patient's laboratory tests and main clinical events
The hospital protocol for COVID‐19 was activated, and the patient was admitted to a dedicated COVID‐19 pathway. On D1, treatment with lopinavir/ritonavir (400 mg/100 mg BID) and hydroxychloroquine (400 mg BID) was initiated and maintained for 7 and 10 days, respectively. CSA and EVL were withdrawn, while prednisolone was increased to 40 mg daily. Despite negative microbiological cultures, empirical broad‐spectrum antibiotic therapy was initiated. Antithrombotic prophylaxis (low molecular weight heparin) was also introduced on D6.
After 7 days of CNI withdrawal, CSA was reinstated and gradually increased to the initial dosage.
Respiratory symptoms were absent until D6, when progressive hypoxia developed with progressive worsening of respiratory function. On D8, a second thoracic CT scan confirmed disease progression (Figure 1B). Laboratory tests revealed interleukin‐6 (IL‐6) serum levels of 147 pg/mL (normal value < 7 pg/mL); PCR rose to 4.2 mg/dL; lymphocyte count fell to 0.34 × 109/L.
The patient was evaluated for treatment with tocilizumab, a recombinant humanized anti‐human IL‐6 receptor monoclonal antibody, and received a single dose of 8 mg/kg intravenously. Following the tocilizumab administration, the patient remained apyretic with substantially stable respiratory function in the subsequent 48 hours. As expected, on D10 IL‐6 rose to 4830 pg/mL.
On D10, the patient's respiratory symptoms worsened, and she was placed on non‐invasive ventilation (NIV) and high‐flow nasal oxygen therapy (HFNO) for 3 days, after which she required intubation for mechanical ventilation and was admitted to the intensive care unit (ICU) (D14).
A new CT showed bilateral, multiple, and subpleural GGO consolidation, and thickening of intralobular septa (crazy‐paving pattern) (Figure 1C).
The patient's data at ICU admission including respiratory data and known negative prognostic indices were fairly similar to the median value of the first 39 consecutive cases admitted to our ICU (Mila Valsecchi MV, Sara Santambrogio SS, Maurizio Bottiroli MB, Roberto Fumagalli RF, unpublished data).
In accordance with acute respiratory distress syndrome (ARDS) ventilation guidelines, the patient was protectively ventilated with low tidal volume (6 mL/kg), PEEP 14 cm H2O after recruitment maneuver, and the driving pressure maintained constantly below 15 cm H2O. 13 Dead space never exceeded 30%. No pronation was needed.
After 72 hours of deep sedation and curarization, weaning was started, and the patient successfully extubated after 10 days of mechanical ventilation (D24).
A CT scan performed on D25 showed pulmonary interstitial fibrosis with no active infection (Figure 1D).
Two consecutive swab tests were negative on D38 and D40. The patient was discharged on D50 with normal kidney graft function, no fever, and peripheral oxygen saturation of 96% in ambient air.
3. DISCUSSION
In Italy, with a population of about 2000 patients/y receiving a kidney transplant in the last 5 years, we would have expected a higher number of immunocompromised patients to have developed COVID‐19. 14 So far, however, only a few cases of COVID‐19 have been reported in KT patients. 3 , 4 , 5
The patient we discuss presented overall clinical characteristics similar to those reported in non‐transplanted COVID‐19 patients. 14 Respiratory data on admission to the ICU were similar to non‐immunosuppressed patients.
Transplanted patients require careful consideration on account of their immunosuppressed status and the risk of graft loss during SARS‐CoV‐2 infection.
We quickly withdrew CSA and EVL, and increased prednisolone to 40 mg/die. Given their anti‐inflammatory effect, steroid adjustments seemed appropriate both to reduce the risk of acute rejection and to mitigate COVID‐19 respiratory symptoms, although its use in COVID‐19 pneumonia remains controversial. 15 , 16
Nonetheless, balancing immunosuppression in COVID‐19 is difficult due to the need to prevent graft rejection while trying to prevent excessive viral replication but also limit immune activation. Reduction or temporary withdrawal of immunosuppressive drugs has been recommended in kidney‐transplanted patients during other viral pandemics. 17
The impact of long‐term immunosuppression on the course of SARS‐CoV‐2 infection is not clear, and CNIs may play a positive role in mitigating the activation of the innate immune response and preventing the evolution of interstitial pneumonia. 18 , 19
We are also aware of the additional risk of infection when increasing steroid therapy, and that the appropriate treatment is a careful balance between the risk of graft loss and the risk of worsening the infection.
According to our ongoing (March 10, 2020) local indication to initiate antiviral treatment as soon as possible, lopinavir/ritonavir and hydroxychloroquine were administered early on for 7 and 10 days, respectively. 20
Similar to coronavirus‐associated SARS, higher IL‐6 levels have been reported in COVID‐19 patients, and the severity of the clinical course—especially in terms of respiratory distress—would seem to be related and proportional to cytokine storm syndrome. 21
The patient received a single dose of tocilizumab in accordance with our local protocol. 20 There is no clear consensus on either the dose or number of administrations. 22 Given the patient's absence of fever, severe lymphopenia (absolute count 270 cells/μcL), concomitant increased immunosuppressive treatment with methylprednisolone, and previous history of severe opportunistic infections, we decide not to administer a second dose in view of the high risk of bacterial infections related to further immunosuppression.
Interestingly, the pneumonia worsened 3 days after tocilizumab administration, highlighting the lack of information regarding the correct timing of administration, which should be possibly have been earlier.
The patient received different treatment purported but not confirmed to be active against SARS‐CoV‐2 infection. We therefore cannot assess which treatment, if any, was more effective. A recent randomized controlled trial has shown lopinavir/ritonavir not to be effective in patients with severe COVID‐19, while data on hydroxychloroquine have been associated with shortening viral replication. 23 , 24
Clinical trial to evaluate tocilizumab in the treatment of COVID‐19 is currently underway in Italy (TOCIVID‐19/NCT04317092), while a preliminary report from China (ChiCTR200029765) has reported its efficacy.
However, its use in solid organ transplant recipients should be carefully considered given the risk of subsequent bacterial infections, as recently reported. 22
After receiving a tailored immunosuppression‐adjusted treatment regimen of lopinavir/ritonavir, hydroxychloroquine, steroid, and tocilizumab, an immunosuppressed KT recipient with severe COVID‐19 pneumonia has recovered. While we agree that strong recommendations cannot be extrapolated from a single case or small series, we nevertheless believe that physicians involved in the care of immunosuppressed patients should clearly and swiftly report data on outcomes with the aim of optimizing treatment protocols, in terms of the timing of immunosuppression modulation and management of antiviral and biologic therapy.
CONFLICT OF INTEREST
We acknowledge no personal conflicts of interest of any of the authors.
AUTHOR CONTRIBUTIONS
The authors reported above meet all of the four listed criteria for authorship: 1‐ substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; 2‐ drafting the work or revising it critically for important intellectual content; 3‐ final approval of the version to be published; and 4‐ agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Contributions of each author can be summarized as follows: AL, MV, and SS involved in the conceptual development, writing the manuscript, analysis, and editing; RDC, MM, AC, LC, VB, and MB involved in analysis and editing; and MP, RF and LDC involved in conceptual development and editing.
Lauterio A, Valsecchi M, Santambrogio S, et al. Successful recovery from severe COVID‐19 pneumonia after kidney transplantation: The interplay between immunosuppression and novel therapy including tocilizumab. Transpl Infect Dis. 2020;22:e13334. 10.1111/tid.13334
REFERENCES
- 1. Zhu L, Xu X, Ma K, et al. Successful recovery of COVID‐19 pneumonia in a renal transplant recipient with long‐term immunosuppression [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? [published online ahead of print 2020]. Am J Transplant . 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seminari E, Colaneri M, Sambo M, et al. SARS Cov2 infection in a renal transplanted patients. A case report [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15902 [DOI] [Google Scholar]
- 5. Bartiromo M, Borchi B, Botta A, et al. Threatening drug‐drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID‐19) [published online ahead of print 2020]. Transpl Infect Dis. 10.1111/tid.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marx D, Moulin B, Fafi‐Kremer S, et al. First case of COVID‐19 in a kidney transplant recipient treated with belatacept [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: a kidney transplant patient with mild COVID‐19 [published online ahead of print 2020]. Transpl Infect Dis;e13296. 10.1111/tid.13296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aslam S, Mehra MR. COVID‐19: Yet another coronavirus challenge in transplantation. J Heart Lung Transpl. 2020;39(5):408‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abadja F, Atemkeng S, Alamartine E, Berthoux F, Mariat C. Impact of mycophenolic acid and tacrolimus on Th17‐related immune response. Transplantation. 2011;92(4):396‐403. [DOI] [PubMed] [Google Scholar]
- 12. Boyarsky BJ, Chiang TP‐Y, Werbel WA, et al. Early impact of COVID‐19 on transplant center practices and policies in the United States [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775‐1786. [DOI] [PubMed] [Google Scholar]
- 14. D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic [published online ahead of print 2020]. Liver Transplant. 10.1002/lt.25756 [DOI] [PubMed] [Google Scholar]
- 15. Lansbury L, Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019;2(2):CD010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid‐organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaza G, Leventhal J, Signorini L, Gambaro G, Cravedi P. Effects of antirejection drugs on innate immune cells after kidney transplantation. Front Immunol. 2019;10:2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lombardy Section Italian Society Infectious and Tropical Diseases . Vademecum for the treatment of people with COVID‐19. Edition 2.0, 13 March 2020. Le Infez Med. 2020;28(2):143‐152. [PubMed] [Google Scholar]
- 21. Ahmadpoor P, Rostaing L. Why the immune system fails to mount an adaptive immune response to a Covid ‐19 infection [published online ahead of print 2020]. Transpl Int. 10.1111/tri.13611 [DOI] [PubMed] [Google Scholar]
- 22. Fontana F, Alfano G, Mori G, et al. Covid‐19 pneumonia in a kidney transplant recipient successfully treated with Tocilizumab and Hydroxychloroquine [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19 [published online ahead of print 2020]. N Engl J Med. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial [published online ahead of print 2020]. Int J Antimicrob Agents;105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


