Abstract
Severe acute respiratory syndrome coronavirus (SARS‐CoV)‐2, a novel coronavirus from the same family as SARS‐CoV and Middle East respiratory syndrome coronavirus, has spread worldwide leading the World Health Organization to declare a pandemic. The disease caused by SARS‐CoV‐2, coronavirus disease 2019 (COVID‐19), presents flu‐like symptoms which can become serious in high‐risk individuals. Here, we provide an overview of the known clinical features and treatment options for COVID‐19. We carried out a systematic literature search using the main online databases (PubMed, Google Scholar, MEDLINE, UpToDate, Embase and Web of Science) with the following keywords: ‘COVID‐19’, ‘2019‐nCoV’, ‘coronavirus’ and ‘SARS‐CoV‐2’. We included publications from 1 January 2019 to 3 April 2020 which focused on clinical features and treatments. We found that infection is transmitted from human to human and through contact with contaminated environmental surfaces. Hand hygiene is fundamental to prevent contamination. Wearing personal protective equipment is recommended in specific environments. The main symptoms of COVID‐19 are fever, cough, fatigue, slight dyspnoea, sore throat, headache, conjunctivitis and gastrointestinal issues. Real‐time PCR is used as a diagnostic tool using nasal swab, tracheal aspirate or bronchoalveolar lavage samples. Computed tomography findings are important for both diagnosis and follow‐up. To date, there is no evidence of any effective treatment for COVID‐19. The main therapies being used to treat the disease are antiviral drugs, chloroquine/hydroxychloroquine and respiratory therapy. In conclusion, although many therapies have been proposed, quarantine is the only intervention that appears to be effective in decreasing the contagion rate. Specifically designed randomized clinical trials are needed to determine the most appropriate evidence‐based treatment modality.
Keywords: COVID‐19, COVID‐19 diagnosis, COVID‐19 management, COVID‐19 treatment, novel coronavirus, SARS‐CoV‐2

Introduction
Severe acute respiratory syndrome coronavirus (SARS‐CoV)‐2, a novel RNA coronavirus from the same family as SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV), was identified in early January 2020 as the cause of a pneumonia epidemic affecting the city of Wuhan, the capital of Hubei province, from where it rapidly spread across China. After infecting and causing the death of thousands of persons in China, the virus has spread, reaching Italy and other European countries [1, 2, 3] and the USA, with the number of confirmed new cases currently increasing every day.
The World Health Organization named the disease coronavirus disease 2019 (COVID‐19) and subsequently declared it a pandemic due to the widespread infectivity and high contagion rate. Human coronaviruses typically cause respiratory and enteric infections [4]. The new coronavirus has become a worldwide health threat [5]: up to 28 March 2020, COVID‐19 has caused the death of 26 495 individuals worldwide and infected more than 570 000 [6]. The SARS‐CoV‐2 infection mainly presents flu‐like symptoms such as fever, cough and asthenia, similar to other coronaviruses [7]. Although severe lung injury has been described at all ages, in some high‐risk individuals, such as the elderly or those affected by multimorbidities, the virus is more likely to cause severe interstitial pneumonia, acute respiratory distress syndrome (ARDS) and subsequent multiorgan failure, which are responsible for severe acute respiratory failure and high death rates. Typically, affected individuals display a variable extent of dyspnoea and radiological signs [8, 9]. Here, we summarize the currently available data on the clinical features and treatment options for COVID‐19.
Methods
Using online databases, we carried out a systematic literature review of the clinical features of and treatments for the new COVID‐19. Key articles were retrieved mainly from PubMed, Google Scholar, MEDLINE, UpToDate, Embase and Web of Science, using the terms ‘COVID‐19’, ‘2019‐nCoV’, ‘coronavirus’ and ‘SARS‐CoV‐2’ as keywords for our search. We included scientific publications from 1 January 2019 to 3 April 2020. Only publications focusing on clinical characteristics of and treatments for SARS‐CoV‐2 were eligible for inclusion. We screened all reference lists of relevant studies in order to identify any missing publications.
All searches as well as title and abstract screening and study selection were performed by two investigators working independently. We resolved any discrepancies through consensus. All articles deemed potentially eligible were retrieved for full‐text review. We limited our search results to publications in English and excluded abstracts from conferences and commentaries.
Virus transmission and epidemiology
The plausible mechanism of interspecies transmission of the virus is not yet fully understood.
Several research groups have independently identified SARS‐CoV‐2 as belonging to the family of β‐coronaviruses, with a genome almost identical to that of bat coronavirus. These studies indicate that bats may serve as the natural host of the virus.
The novel coronavirus uses the same receptor as SARS‐CoV [angiotensin‐converting enzyme 2 (ACE2)] and mainly spreads through the respiratory tract. Human‐to‐human aerosol transmission is undoubtedly the main source of contagion, which happens mainly through contaminated droplets, hands or surfaces. Virus particles, which are present in secretions from an infected person’s respiratory system, infect others through direct contact with mucous membranes [10] with a median incubation period of between 2 and 12 days (median 5.1 days) [11]. Of note, virus transmission by asymptomatic or affected individuals during the incubation period has been described in detail [12, 13].
An analysis of 22 studies revealed that human coronaviruses, including MERS‐CoV and endemic human coronavirus, can persist on surfaces such as metal, glass or plastic for up to 9 days, but can also be efficiently inactivated within 1 min through a surface disinfection using 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite [14]. Furthermore, most of the available evidence supports the thesis that social distancing of 1.5 m is enough to prevent airborne transmission.
It appears that transmission is possible for approximately 8 days after symptoms appear. Patients may continue to show a positive pharyngeal swab for several weeks after remission of symptoms [15]; however, viable virus cannot be detected after about 8 days of disease, suggesting that prolonged polymerase chain reaction (PCR) test positivity probably does not correlate with clinical transmission.
Guidelines from the US Centers for Disease Control and Prevention do not precisely define the length of isolation for patients [16]. It is reasonable to suggest that isolation is no longer necessary after two consecutive negative real‐time (RT)‐PCR tests at an interval of at least 24 h and in the absence of relevant clinical or epidemiological criteria. However, considering the rate of false‐negative results with throat swabs [17], this recommendation should be considered with extreme caution.
The following practical suggestions may minimize the risk of diagnostic errors. First, diagnostic accuracy may be improved by combining clinical evidence with results from chest computed tomography (CT) and RT‐PCR. Secondly, RT‐PCR results should be interpreted according to epidemiological, clinical and radiological factors. Finally, upper (or lower) respiratory tract specimens should be re‐tested in patients with negative RT‐PCR and high suspicion or probability of infection [18].
The current data suggest that the average number of individuals an affected person can infect [i.e. the reproduction number (R0)] is around 2.5–2.9 [19]. R0 here reflects both the virulence of the virus and the number of social contacts and mixing patterns amongst the population relevant to the dissemination of the disease.
Specific settings and environments should be considered at higher risk of virus dissemination, and in particular, transmission in healthcare settings is an important consideration. In the case series reported by Wang et al. [20], 57 of 138 infections occurred in hospital settings, 40 of which involved healthcare providers. Similarly, residential and nursing homes along with other community dwelling facilities were responsible for local epidemic clusters in Italy, Spain and the USA [21].
General hygiene precautions are crucial in order to minimize the risk of contamination. Wearing masks, gowns, eye protection and gloves, especially for medical staff, are also recommended [22]. Because an infected healthcare provider is a potential vehicle for virus dissemination, it is clear that avoiding the risk of infection amongst healthcare providers is essential. Moreover, a sudden decrease in the number of healthcare providers because of quarantining or isolation would critically overload the healthcare system.
The clinical presentation of COVID‐19 begins within 14 days of exposure; however, in most cases symptoms present after about 5 days and symptom onset is within 11.5 days in 97.5% of individuals [11].
In China, this outbreak showed a cumulative attack rate of 0–11% [7]. When compared to H1N1 influenza, which shared the same transmission pathways, the cumulative attack rate of COVID‐19 is 50‐fold higher. This underlines the necessity of quarantine and social distancing measures, which worldwide governments have chosen to take. Indeed, isolation is still the most effective method for containing the spread of COVID‐19 [23].
We believe that containment of the outbreak should remain the cornerstone of COVID‐19 treatment. The cost of short‐term containment measures would be far lower than the cost of long‐term viral spread. Containment of individual cases might prove impossible or ineffective in the long term; nevertheless, public health measures would definitely be effective in delaying the onset of widespread community transmission and reducing the peak incidence and impact on public services. These measures could also give healthcare systems time to scale up their response, reducing direct and indirect deaths, and slow down the global spread until effective therapies or vaccines are found [24].
COVID‐19 symptoms, course and prognosis
The symptoms of COVID‐19 vary amongst individuals, ranging from asymptomatic infection to severe respiratory failure [25]. An Italian population cohort study conducted in the town of Vò Euganeo by Dr. Lavezzo and colleagues, 2020 (unpublished data) showed that around 50–75% of individuals with positive RT‐PCR throat swab results remain asymptomatic, whilst others develop mild flu‐like symptoms and a further small percentage (about 10% of all symptomatic patients) present dyspnoea, severe interstitial pneumonia, ARDS and multiorgan dysfunction. The vast majority of individuals with symptoms and more severe clinical patterns had one or more coexisting medical conditions, such as hypertension, diabetes and cardiovascular disorders, with elevated case fatalities amongst elderly and frail patients [26, 27]. Common symptoms of the disease are fever, cough, fatigue, slight dyspnoea, sore throat, headache and conjunctivitis. [28, 29] It is therefore difficult to differentiate COVID‐19 from other respiratory diseases [30, 31, 32]. Gastrointestinal involvement was reported in a lower percentage of cases, with diarrhoea, nausea and vomiting.
Li et al. [33] hypothesized that SARS‐CoV‐2 could have neuroinvasive potential as viral entry into the central nervous system may partially contribute to the development of respiratory failure in some patients. The reported hyposmia and dysgeusia experienced by individuals with COVID‐19 could also indicate a potential neurotropism of this virus that may invade the olfactory nerve and bulb or, alternatively, the sensory fibres of the vagus nerve, which from the brainstem innervates different organs of the respiratory tract, including the larynx, trachea and lungs [34]. However, the neuroinvasive potential of SARS‐CoV‐2 remains poorly understood and warrants further investigation [35].
The course of the infection is mild or asymptomatic in about 80–90% of cases. It becomes serious only in around 10% of cases, with dyspnoea, hypoxaemia and extensive (>50%) radiological involvement of the lung parenchyma. A critical condition develops in around 5% of cases, with respiratory failure, pneumonia, shock, multiorgan failure and, in the most serious cases, death, which is almost always caused by progression to ARDS and multiorgan failure [25, 36, 37]. The development of respiratory failure without subjective perception of dyspnoea (‘silent hypoxaemia’) has also been reported [38]. In these cases, hypocapnia caused by compensatory hyperventilation is an accompanying finding.
The mortality rate is variable, ranging from 2% to 5%; variability amongst different studies is probably due to different patient features and/or infection prevalence rates and is affected by the relative number of diagnostic tests performed in symptomatic individuals [36]. It is also possible that rapid saturation of intensive care facilities may have affected mortality rates, especially in epidemic hotspots.
The typical course of severe pathology includes the appearance of overt dyspnoea 6 days after the onset of flu‐like symptoms, hospitalization after a further 8 days and the need for tracheal intubation 10 days after hospitalization [39]. Table 1 summarizes the prevalence of reported symptoms.
Table 1.
Symptoms observed in various cohorts of patient
| Guan et al. [40] | Chen et al. [28] | Shi et al. [67] | Huang et al. [47] | Yang et al. [29] | |
|---|---|---|---|---|---|
| Patients (n) | 1081 | 99 | 21 | 41 | 52 |
| Fever | 473 (44%) | 82 (83%) | 18 (86%) | 40 (98%) | 46 (89%) |
| Dyspnoea | 205 (19%) | 31 (31%) | 9 (43%) | 22 (54%) | 33 (64%) |
| Cough | 745 (69%) | 81 (82%) | 15 (71%) | 31 (76%) | 40 (77%) |
| Sputum | 370 (34%) | – | 3 (14%) | 11 (27%) | – |
| Rhinorrhoea | 53 (5%) | 4 (4%) | 5 (24%) | – | 3 (6%) |
| Sore throat | 153 (14%) | 5 (5%) | – | – | – |
| Headache | 150 (14%) | 8 (8%) | 2 (10%) | 2 (5%) | 3 (6%) |
| Diarrhoea | 42 (4%) | 2 (2%) | 1 (5%) | 1 (2%) | – |
| Nausea/vomiting | 55 (5%) | 1 (1%) | 2 (10%) | – | 2 (4%) |
| Myalgia | 164 (15%) | 11 (11%) | – | – | 6 (12%) |
At present, it is unclear how many affected individuals need hospitalization. Amongst hospitalized patients, around 10–20% are admitted to the intensive care unit (ICU), 3–10% require intubation and 2–5% die [40].
It has been reported that mortality due to COVID‐19 is around 3% [41], which therefore appears be lower than for SARS‐CoV (10%) and MERS‐CoV (35%). However, considering the recent and rapid spread of COVID‐19, it is still too early to determine the actual mortality rate of the condition. Current evidence indicates that the main risk factors for poor outcome include age, ischaemic heart disease, hypertension, diabetes mellitus and chronic lung disease [42].
Diagnosis
RT‐PCR is a diagnostic test that uses nasal swab, tracheal aspirate or bronchoalveolar lavage (BAL) specimens. The primary, and preferred, method for diagnosis is the collection of upper respiratory samples via nasopharyngeal and oropharyngeal swabs. The use of bronchoscopy as a diagnostic method for COVID‐19 is not recommended as the aerosol that is generated poses a substantial risk for both patients and healthcare staff. Bronchoscopy can be considered only for intubated patients when upper respiratory samples are negative and other diagnostic tools would significantly change the clinical management. However, bronchoscopy may be indicated when clinical and safety criteria are met and in the case of uncertain diagnosis [43]. Alternatively, tracheal aspiration and nonbronchoscopic BAL can be used to collect respiratory specimens in intubated patients [44].
SARS‐CoV‐2 RNA has been extracted from upper and lower respiratory tract specimens, and the virus has been isolated in a cell culture of upper respiratory tract secretions and BAL specimens; however, limited RNA data are available. In one case series, Zou et al. found that the levels of SARS‐CoV‐2 RNA were higher in samples collected from the upper respiratory tract (as demonstrated by lower cycle threshold values in the nose) and in the first 3 days after symptom onset, and high levels of SARS‐CoV‐2 RNA were also found in samples collected from upper respiratory tract samples from an asymptomatic patient [45].
Several studies have shown that SARS‐CoV‐2 RNA can also be detected in blood and stool specimens [46, 47, 48, 49]. How long SARS‐CoV‐2 RNA is present in the upper and lower respiratory tracts and in extrapulmonary specimens remains undetermined. It is plausible that viral RNA would be detectable for weeks, as observed in some cases of infection with SARS‐CoV or MERS‐CoV. Viable SARS‐CoV has been isolated from respiratory, blood, urine and stool samples [50, 51, 52, 53, 54, 55, 56, 57, 58].
The specificity of the RT‐PCR test seems to be very high, although there may be false‐positive results due to swab contamination, especially in asymptomatic patients. The sensitivity rate is not clear, but is estimated to be around 66–80% [59]. Test validity in asymptomatic individuals who have been in close contact with symptomatic persons is even less clear; the rate of positivity could reach 50% without any evidence of symptoms or proven infection [60].
A single negative test does not exclude SARS‐CoV‐2 infection, especially in highly exposed persons, if the test is performed using a nasopharyngeal swab specimen and at the beginning of the infection. In this case, it may be advisable to repeat the test or collect a deeper respiratory tract sample, such as BAL.
Laboratory test results
The most common laboratory abnormalities reported on admission amongst hospitalized patients with pneumonia included leucopenia (9–25%) or leucocytosis (24–30%), lymphopenia (63%) and elevated levels of alanine aminotransferase and aspartate aminotransferase (37%) [46, 48]. Amongst 1099 COVID‐19 patients, lymphocytopenia was present in 83%; in addition, 36% had thrombocytopenia and 34% had leucopenia [40]. A mild thrombocytopenia, hypertransaminasaemia and an increase in lactate dehydrogenase have also been reported [47].
Increased inflammation indices, usually including reduced procalcitonin and increased C‐reactive protein (CRP) levels, are associated with clinical severity. Young et al. observed an average CRP level of 1.1 mg/dL in patients with normal percentage oxygen saturation (SatO2) and of 6.6 mg/dL in hypoxaemic patients [48]. Moreover, Ruan et al. observed a correlation between CRP and mortality risk [61]. Increased troponin was also reported in 7% of patients who subsequently died because of fulminant myocarditis [62]. Troponin appears to be a strong prognostic indicator of mortality. Finally, it was noticed that D‐dimer and ferritin levels were usually high in hospitalized patients.
Radiological findings
Typical CT findings in individuals with COVID‐19 were ground‐glass opacities, particularly on the peripheral and lower lobes, and bilateral multiple lobular and subsegmental areas of consolidation, especially in ICU patients [10]. The number of lung segments involved was found to be related to disease severity. These opacities tended to flow together and thicken with progression of the disease. Nontypical CT findings included pleural effusion (only about 5%), masses, cavitations and lymphadenopathies; therefore, these would suggest alternative diagnoses [63]. Figure 1 illustrates typical CT patterns in COVID‐19 patients.
Fig. 1.
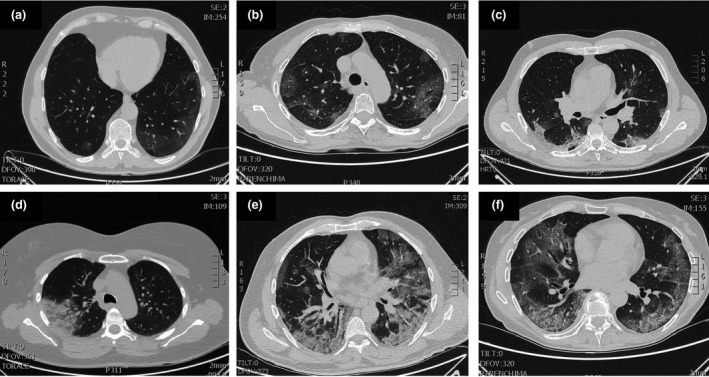
Typical patterns of COVID‐19 at CT imaging. Ground glass shadows (early stage). Ground‐glass opacities. Ground glass nodules and subpleural consolidation. Focal consolidation. Multifocal consolidation. Multifocal consolidation with honeycomb (end stage).
In one study, the time period from symptom onset to initial CT scan was evaluated and the authors found that 56% of patients who presented symptoms within 2 days had normal CT images [64]. CT sensitivity seems to be high in patients with positive RT‐PCR (86–97% in different case studies) [60] and lower in patients with only constitutional and nonrespiratory symptoms (about 50%) [63].
Conventional chest X‐ray sensitivity is lower at around 59% [64]. Ultrasound has been used as a diagnostic tool in a very limited number of cases. Ultrasound has a very low specificity, and, despite being affected by factors such as disease severity, patient weight and operator skill, sensitivity is estimated to be around 75% [65]. Nevertheless, ultrasound may play a role in monitoring the progression of the disease through the detection of interstitial lung disease features, such as B lines and subpleural consolidations. Fig. 2 shows three different interstitial presentations on ultrasound in COVID‐19 patients.
Fig. 2.
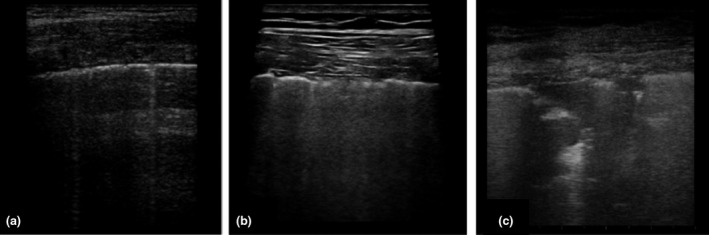
Patterns of COVID‐19 at chest ultrasound. Early bilateral multifocal areas of interstitial syndrome. Interstitial pneumonia characterized by interstitial syndrome with B lines and preserved sliding sign. Advanced, organized pneumonia with interstitial syndrome associated with multiple subpleural consolidations and reduced sliding sign.
CT and ultrasound findings appear to be superimposable; CT seems to be more precise in detecting apical intraparenchymal lesions, whilst ultrasound can identify the smallest subpleural lesions and pleural effusions. Sensitivity for subpleural lesions increases when a linear probe is used. The main ultrasound findings include isolated or confluent B lines, and irregular or interrupted pleural line thickening with dynamic air bronchogram [65]. Most of these pathological findings are located in lower and posterior areas. It is possible to scan in colour Doppler mode in order to detect a reduced blood supply in the lesions (usually increased in other inflammatory diseases).
At present, the best radiological strategy remains undefined. The use of CT for all patients appears to be unreasonable in terms of time, cost and radiation exposure, especially as the management and therapeutic approach would not depend substantially on the results. We suggest that CT scanning should be reserved for patients with an undefined clinical picture, as well as differential diagnosis [66, 67].
Clinical management
Currently, there are no registered drugs to treat COVID‐19 disease and a vaccine is not available [68, 69, 70]. Management is based mainly on supportive therapy and on treating the symptoms and trying to prevent respiratory failure.
It is fundamental to ensure patient isolation in order to avoid transmission to other patients, family members and healthcare providers. Quarantine measures must be taken to isolate infected individuals, both symptomatic and asymptomatic, and anyone who may have been in contact with them [71]. Entire populations must limit social contact and minimize the time spent outside [72]. In mild cases, self‐isolation at home is the best option, whilst maintaining adequate hydration and nutrition and treating symptoms such as fever, sore throat or cough. Thus, hospital beds can be available for severe cases [31].
Most of the data available for pharmacological treatments derive from medications used during the SARS‐CoV or MERS‐CoV pandemics or from in vitro observations [73, 74]. Several clinical trials of possible treatments for COVID‐19 are underway, based on antiviral, anti‐inflammatory and immunomodulatory drugs, cell therapy, antioxidants and other therapies [75]. The inflammatory pathway involved in COVID‐19 is shown in Fig. 3, and Table 2 provides an overview of the current treatments available to manage the disease.
Fig. 3.
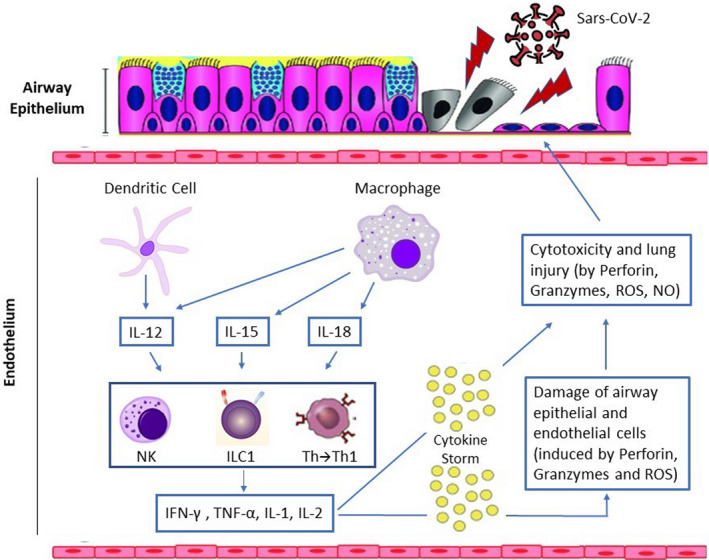
Virus‐induced inflammation pathway. Immune cells are sequentially activated to limit virus dissemination. Dendritic cells and macrophages act as first‐line antigen‐presenting cells which, following virus antigen recognition, produce cytokines, including interleukin (IL)‐12, IL‐15 and IL‐18. Their interaction determines the chemotaxis and activation of natural killer (NK) cells, the recruitment of Group 1 innate lymphoid cells (ILC1) and the differentiation of T helper (Th) lymphocytes into Type 1 helper (Th1) cells. The latter are associated with an increased expression of cytokines, including interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α, IL‐1 and IL‐2, with consequent activation of NK cells, secreting perforin, granzymes, reactive oxygen species (ROS), nitric oxide (NO) and cytotoxic T lymphocytes in order to kill the virus. Excess neutrophils and persistently activated macrophages cause extensive damage to the lung epithelium and endothelium, resulting in an alveolar capillary barrier. The disruption of this barrier allows protein‐rich fluid to enter the alveoli, causing fluid accumulation in alveolar spaces (noncardiogenic pulmonary oedema) which interferes with gas exchange.
Table 2.
Severity‐dependent protocol to manage COVID‐19
| Phenotype No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Symptoms and clinical features | Fever; mild respiratory symptoms (if any); SpO2 ≥ 93% on ABG; no chest X‐ray findings | Fever + SpO2 <93% on ABG OR X‐ray opacities | Fever; severe hypoxia (SpO2 < 88%) on ABG BUT patient responsive to high flow of O2 (SpO2 ≥ 93% with O2 10–15 L/min) | High probability of ARDS evolution; NIV needed to maintain acceptable SpO2 levels | ARDS |
| Illness severity | Mild illness | Moderate illness | Pre‐critical illness | Critical illness | |
| Allocation | Self‐isolation at home | Hospitalization | Hospitalization in sub‐intensive care unit | Hospitalization in sub‐intensive or intensive care unit | Hospitalization in intensive care unit |
| Vital support/ Respiratory treatment | Symptomatic treatment |
Symptomatic treatment; oxygenation through nasal cannula, face mask or high‐flow nasal cannula EVIDENCE: effective in maintaining SpO2 ≥ 93 % [103, 104, 105, 106, 107] |
Oxygenation through nasal cannulas, face mask or high‐flow nasal cannulas to reach SpO2 target ≥ 93% If target not reached after 30 min, evolution to PHENOTYPE 4 |
Start CPAP/NIV with SpO2 target> 93% EVIDENCE: Effective in maintaining spO2 ≥ 93%[103, 104, 105, 106, 107] If target not reached after 2 h, evolution to PHENOTYPE 5 |
Tracheal intubation; mechanical ventilation with high PEEP values and repeated positioning EVIDENCE: effective in increasing oxygenation [104, 105, 106, 107, 108] |
| Antiviral therapy | None |
Lopinavir/ritonavir 400/100 mg BID orally (duration: according to clinical evolution) EVIDENCE: reduces viral load (low‐quality evidence) [83, 84, 85, 86] |
Lopinavir/ritonavir 400/100 mg BID orally (duration: according to clinical evolution) OR Remdesivir 200 mg iv followed by 100 mg OD (5–10 days according to clinical evolution) EVIDENCE: reduces viral load (low‐quality evidence) [81, 82, 83] |
||
| Other treatments |
Self‐isolation at home |
Chloroquine 500 mg BID orally (5–20 days according to clinical evolution) OR Hydroxychloroquine 200 mg BID orally (5–20 days according to clinical evolution). EVIDENCE: reduces viral load (low‐quality evidence) [93, 94, 95, 96, 97, 98, 99, 100, 102] |
|||
SpO2, peripheral oxygen saturation; BID, twice daily; OD, once a day; iv, intravenous; ABG, arterial blood gas; ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; NIV, noninvasive ventilation; PEEP, positive end‐expiratory pressure.
There is no evidence that antibiotic prophylaxis can prevent bacterial superinfection, and indeed no evidence of a diagnostic role of procalcitonin in COVID‐19 patients. Superinfections are more likely to develop in a hospital environment than at home. Ruan et al. reported a death rate of 16% in COVID‐19 patients who had contracted secondary infections [61].
There is also no conclusive evidence regarding the use of steroids. Most available data are from descriptive studies and shared experience. The use of steroids to treat SARS‐CoV and MERS‐CoV cases was associated with increased mortality, secondary infections and complications such as psychosis, hyperglycaemia, delayed viral clearance and increased mutation rate of the pathogen. Because there is no conclusive evidence to support steroid use, it is necessary to cautiously evaluate steroid use on a case‐by‐case basis, balancing risks and benefits. Should steroid‐based therapy become necessary, it is mandatory to use the lowest possible dosage and only for a short period of time [76, 77, 78, 79].
Anticoagulation therapy is recommended in patients with early‐stage COVID‐19, especially when the D‐dimer value is 4 times higher than normal. Infection, inflammation and other disease‐related factors can cause overactivation of coagulation, increasing the risk of augmented ischaemic events and disseminated intravascular coagulation [80].
Antiviral drugs
The efficacy of specific antiviral agents to treat COVID‐19 has been shown both in vitro and in animal models, as well as from anecdotal evidence from human patients. These studies are based almost exclusively on experience with SARS‐CoV and MERS‐CoV [81, 82, 83, 84, 85, 86, 87]. The Italian Society of Infective and Tropical Diseases recommends administering antiviral agents to patients with a proven diagnosis of COVID‐19 and mild symptoms [88]. However, antiviral agents should be avoided in the presence of comorbidities and increased risk of mortality or in individuals with moderate or severe symptoms of COVID‐19.
Remdesivir was successfully used in several COVID‐19 patients in China [81]. As a nucleotide analogue, remdesivir acts through incorporation into the nascent viral RNA chain and subsequently causes its premature termination. Remdesivir has been reported to be active in preclinical studies of SARS‐CoV and MERS‐CoV infections by acting on the viral polymerase of coronaviruses [82]. A North American study of MERS‐CoV in mice has shown the effectiveness of remdesivir in reducing viral load and improving lung function parameters [83]. Clinical efficacy trials of the use of remdesivir in COVID‐19 patients are currently underway, both in China and the USA.
The second‐generation antiretroviral drug combination lopinavir/ritonavir inhibits viral protease. The combination is widely available and drug interaction and safety profiles are well established. The efficacy of lopinavir/ritonavir against SARS‐CoV has been demonstrated [84], and these drugs also seem to reduce the viral load in COVID‐19 patients [85, 86]. However, the clinical evidence for this combination therapy remains limited, as suggested by case reports [48, 85, 87]; moreover, Cao et al. [89] observed no clinical benefit of lopinavir/ritonavir beyond standard care. Multiple randomized controlled trials, however, are ongoing in China. The suggested dosage is 400/100 mg twice daily (BID); adjustment based on glomerular filtration rate is not required but monitoring of transaminases is often useful. Common side effects of lopinavir/ritonavir include nausea, diarrhoea and insomnia, and numerous drug interactions have been reported.
Chloroquine/hydroxychloroquine
Chloroquine and hydroxychloroquine are used for the treatment of malaria and amoebiasis. They both show a good tolerability profile. Various studies have demonstrated chloroquine activity in vitro and in animal models against SARS‐CoV [90, 91] and avian influenza [92]. Their antiviral efficacy seems to be explained by an increase in endosomal pH which is necessary for fusion between the virus and the host cell; they also seem to interfere with the ACE2 cell receptor and have immunomodulatory activity. We have also found evidence of their efficacy in COVID‐19 patients [87, 93, 94, 95, 96, 97, 98]. The suggested dosages are 500 mg BID for chloroquine and 200 mg BID for hydroxychloroquine. For optimal treatment, a loading dose should be administered and followed by a maintenance dose [99]. Yao et al. showed that, in vitro, hydroxychloroquine is more potent than chloroquine in inhibiting SARS‐CoV‐2 [10]. Common side effects of these drugs are nausea, vomiting, diarrhoea, abdominal pain, headache and visual and extrapyramidal disorders. Monitoring of blood count and QT interval is mandatory, due to their well‐known arrhythmogenic cardiotoxicity [11]. Data on the efficacy of chloroquine and hydroxychloroquine remain inconclusive, and further studies are warranted. Several issues need to be clarified such as the stage of COVID‐19 disease at which these medications could provide the best therapeutic benefit, or whether they may play a role in disease prophylaxis for high‐risk patients and healthcare providers [12]. Finally, it seems that antimalarial medications may act synergistically with macrolides (e.g. azithromycin) for enhanced antiviral effect but, once again, the existing evidence is limited [97] and studies had several limitations (e.g. lack of randomization and covariate‐adjusted analysis, and potential selection bias).
Table 2 shows a severity‐dependent therapeutic protocol for the treatment of COVID‐19 disease.
Respiratory therapy
Oxygen therapy will be required if hypoxia is present (SatO2 < 93%) or if symptoms of respiratory distress become evident. Oxygen therapy is generally administered through a (high‐flow) nasal cannula, a face mask or noninvasive ventilation (preferably using a continuous positive airway pressure helmet or full‐face interface, avoiding a nasal mask and nasal pillow due to the risk of infected aerosol spread) [103, 104, 105, 106, 107]. Arterial SatO2 must be monitored constantly during oxygen therapy.
If a sufficiently high arterial O2 level (SatO2 93–96%) is not reached, and if acute lung injury develops (ratio of arterial partial pressure of oxygen to fractional inspired oxygen ≤ 200 mmHg), invasive mechanical ventilation and intubation are required [14]. Tracheal intubation should not be delayed in patients with a low oxygenation index, worsening of respiratory distress symptoms or multiorgan failure whilst administering noninvasive O2 therapy [104, 105, 106, 107, 108]. Advanced techniques should be considered for invasive mechanical ventilation, such as pressure‐ and volume‐limited ventilation, positive end‐expiratory pressure, use of neuromuscular blocking agents and prone positioning [16]. Most critically ill patients can respond well to prone positioning, with a rapid increase in oxygenation and lung mechanics [107, 108]. Extracorporeal membrane oxygenation could be a viable treatment option [19] for COVID‐19 patients affected by severe ARDS and not responding to the above‐mentioned protocols.
Possible future therapeutic targets
There is growing interest in the use of specific anti‐inflammatory molecules such as tocilizumab, a monoclonal antibody against IL‐6R. Tocilizumab was used in Wuhan to treat 272 COVID‐19 patients and is being investigated in an ongoing national multicentre clinical trial in Italy. Although promising, the currently available data are still too limited to draw any conclusion about the viability of these therapies.
Other potential anti‐inflammatory therapies might include anti‐IL‐17, interferon and treatment with mesenchymal stromal cells which reduce inflammation and stimulate regeneration of tissues affected by ARDS [110].
The amplification of anti‐2019nCoV‐specific T lymphocytes may be another feasible option [111]. Other potentially interesting but purely speculative options at present include molecules acting on the Th1‐mediated inflammatory cascade, such as canakinumab (a human monoclonal antibody targeting IL‐1β) or roflumilast (a selective, long‐acting inhibitor of the enzyme phosphodiesterase‐4 which is already used to control neutrophilic inflammation in patients with chronic obstructive pulmonary disease) [112, 113].
Development of therapeutic antibodies is increasingly heading towards the mimicry of normal protective antibody responses elicited in the context of innate receptor engagement (including Fc, Toll‐like and complement receptors). Treatments with multiple monoclonal antibodies and immunostimulants are being considered for neutralization of variant strains, which may include SARS‐CoV‐2 [114]. Some studies have focused on inhibitors of the link between the spike (S) protein of the virus and ACE2 [115, 116, 117, 118]: angiotensin receptor 1 blockers (sartanics) [119], emodin, promazine [120, 121], furin (a serine endoprotease) and monoclonal antibodies against the S1 domain of the S protein [122].
An interesting strategy would be targeting the structural genes for the S protein or envelope or membrane proteins with small interfering RNAs [123]. Broad‐spectrum antiviral agents, such as dsRNA‐activated caspase oligomerizer, are known to cause selective apoptosis of virus‐containing host cells; this may be another promising strategy, but only if associated with other therapies as such antivirals cannot block the virus from entering the cell nor disrupt the viral nucleic acid. This path should be evaluated in combination with thiopurine compounds, naphthalene inhibitors, protease inhibitors, zinc or mercury [123].
Furthermore, the use of bradykinin receptor B1 and B2 antagonists may be a novel strategy to treat the bradykinin‐dependent local lung angioedema caused by COVID‐19. In addition, this pathway may be indirectly responsive to anti‐inflammatory agents or neutralizing strategies for anti‐S‐antibody‐induced effects, but these antagonists alone are unlikely to reverse all the pulmonary oedema [124].
Another interesting option that is currently been evaluated is passive immunotherapy with the use of plasma from convalescent patients [125]; however, caution is warranted. Patients who developed the anti‐S‐neutralizing antibody early in the disease course were found to have a higher mortality risk from COVID‐19, and a worsening of pulmonary disease has already been demonstrated in SARS‐CoV infection [126, 127].
It is hoped that a vaccine will become available, but successful development and regulatory approval of a vaccine will take many months.
Conclusion
The global health and economic consequences of the SARS‐CoV‐2 pandemic are severe. Although many therapies have been suggested, at present there are no specific options capable of treating COVID‐19 disease or preventing SARS‐CoV‐2 infection. The only intervention currently viable and proven to decrease the contagion rate seems to be strict quarantine measures for the general population.
Specifically designed randomized clinical trials are urgently needed to determine the most appropriate evidence‐based treatment modality to reduce the spread of this disease and prevent the burden of any future outbreak.
Conflict of interest
The authors declare no affiliations with or involvement in any organization or entity with any financial or nonfinancial interest related to this manuscript.
Author contribution
Giuseppe Pascarella: Conceptualization (equal); Data curation (equal); Methodology (equal); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Alessandro Strumia: Conceptualization (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Chiara Piliego: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Federica Bruno: Data curation (equal); Investigation (equal); Resources (equal); Writing‐original draft (equal). Romualdo Del Buono: Data curation (equal); Methodology; Resources (equal); Supervision; Validation (equal); Writing‐review & editing. Fabio Costa: Conceptualization; Data curation (equal); Project administration (equal); Resources (equal); Supervision. Simone Scarlata: Conceptualization; Data curation (equal); Investigation (equal); Methodology; Resources (equal); Supervision; Validation (equal). Felice Eugenio Agrò: Conceptualization; Data curation; Formal analysis (equal); Project administration (equal); Resources; Supervision; Validation.
Acknowledgements
We thank Damiana Mancini for excellent artwork and graphic support and the Italian Pleural Hub Community for fruitful discussions and medical insight. This work is dedicated to every healthcare provider fighting this pandemic, and to our COVID‐19 patients, their families and loved ones. This study was carried out in memory of our colleagues who lost their lives due to COVID‐19.
Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE (Campus Bio Medico University and Teaching Hospital, Rome; Humanitas Mater Domini Hospital, Castellanza; and Campus Bio Medico University and Teaching Hospital, Rome, Italy). COVID‐19 diagnosis and management: a comprehensive review (Review). J Intern Med 2020; 288:192–206. 10.1111/joim.13091
References
- 1. Remuzzi A, Remuzzi G. COVID‐19 and Italy: what next? The Lancet 2020; 395: 1225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holshue ML, DeBolt C, Lindquist S et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382: 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernard Stoecklin S, Rolland P, Silue Y et al. First cases of coronavirus disease 2019 (COVID‐19) in France: surveillance, investigations and control measures, January 2020. Eurosurveillance 2020; 25: 2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Zheng X, Tong Q et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol 2020; 92: 491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahase E. Covid‐19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 2020; 368: m1036. [DOI] [PubMed] [Google Scholar]
- 6. WHO . Novel coronavirus situation dashboard. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 7. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis 2020; 94: 44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu K, Chen Y, Lin R, Han K. Clinical feature of COVID‐19 in elderly patients: a comparison with young and middle‐aged patients. J Infection 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lake MA. What we know so far: COVID‐19 current clinical knowledge and research. Clin Med (Lond) 2020; 20: 124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adhikari SP, Meng S, Wu YJ et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lauer SA, Grantz KH, Bi Q et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Int Med 2020; 172: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothe C, Schunk M, Sothmann P et al. Transmission of 2019‐nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 2020; 382: 970–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu P, Zhu J, Zhang Z, Han Y. A Familial cluster of infection associated with the 2019 novel coronavirus indicating possible person‐to‐person transmission during the incubation period. J Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lan L, Xu D, Ye G et al. Positive RT‐PCR test results in patients recovered from COVID‐19. JAMA 2020; 323: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prevention CfDCa . Discontinuation of Home Isolation for Persons with COVID‐19 (Interim Guidance). Atlanta, Georgia: Center for Disease Control and Prevention, 2020. [Google Scholar]
- 17. Woelfel R, Corman VM, Guggemos W et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel‐associated transmission cluster. medRxiv 2020. [Google Scholar]
- 18. Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID‐19). Clin Chem Lab Med 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19. Peng PWH, Ho P‐L, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth; 124: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Adamo H, Yoshikawa T, Ouslander JG. Coronavirus Disease 2019 in Geriatrics and Long‐term Care: The ABCDs of COVID‐19. J Am Geriatr Soc 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019‐nCoV) patients. Can J Anaesth 2020; 67: 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou G, Chi C. A model simulation study on effects of intervention measures in Wuhan COVID‐19 epidemic. Medrxiv 2020. [Google Scholar]
- 24. Wilder‐Smith A, Chiew CJ, Lee VJ. Can we contain the COVID‐19 outbreak with the same measures as for SARS? Lancet Infect Dis 2020; 20: e102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He F, Deng Y, Li W. Coronavirus disease 2019 (COVID‐19): What we know? J Med Virol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Zheng Y, Gou X et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. International journal of infectious diseases. IJID 2020; 94: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li B, Yang J, Zhao F et al. Prevalence and impact of cardiovascular metabolic diseases on COVID‐19 in China. Clin Res Cardiol 2020; 109: 531–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020; 395: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Resp Med 2020; 8: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li LQ, Huang T, Wang YQ et al. 2019 novel coronavirus patients' clinical characteristics, discharge rate and fatality rate of meta‐analysis. J Med Virol 2020; 92: 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singhal T. A Review of Coronavirus Disease‐2019 (COVID‐19). Indian J Pediatr 2020; 87: 281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol 2020; 92: 548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 2020; 92: 552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desforges M, Le Coupanec A, Dubeau P et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2020; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun T, Guan J. Novel coronavirus and central nervous system. Eur J Neurol 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 37. Xu Z, Shi L, Wang Y et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Resp Med 2020; 8: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID‐19 epidemic in China. Intens Care Med 2020; 46: 579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bouadma L, Lescure F‐X, Lucet J‐C, Yazdanpanah Y, Timsit J‐F. Severe SARS‐CoV‐2 infections: practical considerations and management strategy for intensivists. Intens Care Med 2020; 46: 579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guan W‐j, Ni Z‐y, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). J Gen Intern Med 2020; 35: 1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W, Xu Y, Gao R et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim Guid. Geneva, Switzerland: World Health Organization site, 2020. [Google Scholar]
- 45. Zou L, Ruan F, Huang M et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382: 1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Du R‐H, Li B et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect 2020; 9: 386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. The Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Young BE, Ong SWX, Kalimuddin S et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA 2020; 323: 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yong Zhang CC, Zhu Shuangli, Shu Chang et al. Isolation of 2019‐nCoV from a stool specimen of a laboratory‐confirmed case of the coronavirus disease 2019 (COVID‐19). China CDC Weekly 2020; 2: 123–4. [PMC free article] [PubMed] [Google Scholar]
- 50. Memish ZA, Assiri AM, Al‐Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS‐CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis 2014; 29: 307–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E et al. Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Med Infect Dis 2020; 101623. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 2015; 386: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chan KH, Poon LLLM, Cheng VCC et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis 2004; 10: 294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cheng PK, Wong DA, Tong LK et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 2004; 363: 1699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hung IF, Cheng VC, Wu AK et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis 2004; 10: 1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peiris JS, Chu CM, Cheng VC et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 2003; 361: 1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu W, Tang F, Fontanet A et al. Long‐term SARS coronavirus excretion from patient cohort. China. Emerg Infect Dis 2004; 10: 1841–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Corman VM, Albarrak AM, Omrani AS et al. Viral shedding and antibody response in 37 patients with middle east respiratory syndrome coronavirus infection. Clin Infect Dis 2016; 62: 477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2019; 2020: 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhuang GH, Shen MW, Zeng LX et al. Potential false‐positive rate among the 'asymptomatic infected individuals' in close contacts of COVID‐19 patients. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 485–8. [DOI] [PubMed] [Google Scholar]
- 61. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Driggin E, Madhavan MV, Bikdeli B et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol 2020; 75: 2352–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for radiologists on COVID‐19: an update—radiology scientific expert panel. Radiology 2020; 200527. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bernheim A, Mei X, Huang M et al. Chest CT findings in coronavirus disease‐19 (COVID‐ 19): relationship to duration of infection. Radiology 2020; 200463. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yi Huang S, Liu Y, Zhang Y, Chuyun Zheng Y, Zheng CZ, Min W, Ming Y, Mingjun H. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19). Research square.
- 66. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad Ali. Coronavirus disease 2019 (COVID‐19): A systematic review of imaging findings in 919 patients. Am J Roentgenol 2019; 2020: 1–7. [DOI] [PubMed] [Google Scholar]
- 67. Shi H, Han X, Jiang N et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20: 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- 69. Pang J, Wang MX, Ang IYH et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019‐nCoV): A systematic review. J Clin Med 2020: 9: 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shanmugaraj B, Malla A, Phoolcharoen W. Emergence of novel coronavirus 2019‐nCoV: Need for rapid vaccine and biologics development. Pathogens 2020; 9: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brooks SK, Webster RK, Smith LE et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. The Lancet 2020; 395: 912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parmet WE, Sinha MS. Covid‐19 — The law and limits of quarantine. N Engl J Med 2020; 382: e28. [DOI] [PubMed] [Google Scholar]
- 73. Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens 2020; 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang Y, Peng F, Wang Ret al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun 2003; 2020: 102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang T, He Y, Xu W, Ma A, Yang Y, Xu K‐F. Clinical trials for the treatment of Coronavirus disease 2019 (COVID‐19): A rapid response to urgent need. Sci China Life Sci 2020; 63: 774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. WHO . Coronavirus disease (COVID‐19) technical guidance: Patient management. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 77. Prevention CfDCa . Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID‐19). Atlanta, Georgia: Center for Disease Control and Prevention, 2020. [Google Scholar]
- 78. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. The Lancet 2020; 395: 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou W, Liu Y, Tian D et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Sign Trans Target Therap 2020; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 9: 727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Al‐Tawfiq JA, Al‐Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID‐19. Travel Med Infect Dis 2020; 101615. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Agostini ML, Andres EL, Sims AC et al. Coronavirus susceptibility to the antiviral remdesivir (GS‐5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018; 9: e00221‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sheahan TP, Sims AC, Leist SR et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun 2020; 11: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chu CM, Cheng VC, Hung IF et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59: 252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lim J, Jeon S, Shin HY et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci 2020; 35: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus‐A possible reference for coronavirus disease‐19 treatment option. J Med Virol 2020; 92: 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Han W, Quan B, Guo Y et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol 2020; 92: 461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. SIMIT . Vademecum per la cura delle persone con malattia da COVID‐19. Prato, Italy: SIMIT, 2020. [Google Scholar]
- 89. Cao B, Wang Y, Wen D et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe covid‐19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis 2006; 6: 67–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vincent MJ, Bergeron E, Benjannet S et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2: 69‐. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yan Y, Zou Z, Sun Y et al. Anti‐malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 2013; 23: 300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends 2020; 14: 72–3. [DOI] [PubMed] [Google Scholar]
- 94. The multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia . Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Chin J Tuberc Respir Dis 2020; 43: 185–8. [DOI] [PubMed] [Google Scholar]
- 95. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Touret F, de Lamballerie X. Of chloroquine and COVID‐19. Antiviral Res 2020; 177: 104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gautret P, Lagier J‐C, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020; 105949. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98. Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 2020; 30: 269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents 2020; 55: 105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Haeusler IL, Chan XHS, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med 2018; 16: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Spinelli FR, Ceccarelli F, Di Franco M, Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS‐CoV‐2 (Covid‐19) pandemic. Ann Rheum Dis 2020; 79: 666–7. [DOI] [PubMed] [Google Scholar]
- 103. Guo YR, Cao QD, Hong ZS et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak ‐ an update on the status. Mil Med Res 2020; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community‐acquired severe respiratory viral infection. Intensive Care Med 2020; 46: 315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rello J, Tejada S, Userovici C, Arvaniti K, Pugin J, Waterer G. Coronavirus disease 2019 (COVID‐19): A critical care perspective beyond China. Anaesth Crit Care Pain Med 2020; 39: 167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fan E, Del Sorbo L, Goligher EC et al. An official American thoracic society/European Society of Intensive Care Medicine/Society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195: 1253–63. [DOI] [PubMed] [Google Scholar]
- 107. Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) infection: an operational recommendation of Peking Union Medical. College Hospital (V2.0). Emerg Microb Infect 2020; 9: 582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Munshi L, Del Sorbo L, Adhikari NKJ et al. Prone position for acute respiratory distress syndrome. A systematic review and meta‐analysis. Ann Am Thorac Soc 2017; 14: S280–s8. [DOI] [PubMed] [Google Scholar]
- 109. MacLaren G, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID‐ 19: the potential role of extracorporeal membrane oxygenation. JAMA 2020; 323: 1245–6. [DOI] [PubMed] [Google Scholar]
- 110. Horie S, Gonzalez HE, Laffey JG, Masterson CH. Cell therapy in acute respiratory distress syndrome. J Thorac Dis 2018; 10: 5607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019‐nCoV: host‐directed therapies should be an option. Lancet 2020; 395: e35–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chakraborty A, Tannenbaum S, Rordorf C et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti‐interleukin‐1beta monoclonal antibody. Clin Pharmacokinet 2012; 51: e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chenoweth AM, Wines BD, Anania JC, Mark Hogarth P. Harnessing the immune system via FcgammaR function in immune therapy: A pathway to next‐gen mAbs. Immunol Cell Biol 2020; 98: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li W, Moore MJ, Vasilieva N et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dimitrov DS. The secret life of ACE2 as a receptor for the SARS virus. Cell 2003; 115: 652–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) spike glycoprotein‐mediated viral entry. Proc Natl Acad Sci USA 2004; 101: 4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kuhn JH, Li W, Radoshitzky SR, Choe H, Farzan M. Severe acute respiratory syndrome coronavirus entry as a target of antiviral therapies. Antivir Ther 2007; 12: 639–50. [PubMed] [Google Scholar]
- 119. Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin‐converting enzyme 2 interaction. Antiviral Res 2007; 74: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang XW, Yap YL. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg Med Chem 2004; 12: 2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sui J, Li W, Murakami A et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A 2004; 101: 2536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Khan S, Siddique R, Shereen MA et al. The emergence of a novel coronavirus (SARS‐CoV‐2), their biology and therapeutic options. J Clin Microbiol 2020; 58: e00187‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. van de Veerdonk FNMGN, van Deuren M, van der Meer JW, de Mast Q, Bruggemann RJ, van der Hoeven H. Kinins and cytokines in COVID‐19: A comprehensive pathophysiological approach. Preprints 2020. [Google Scholar]
- 125. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol 2020; 92: 479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fu Y, Cheng Y, Wu Y. Understanding SARS‐CoV‐2‐mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sinica 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu L, Wei Q, Lin Q et al. Anti‐spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight 2019; 4: e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]


