Abstract
A 48‐year‐old woman with a past medical history of migraines and hyperlipidemia presented due to severe retrosternal chest pain with no other associated signs or symptoms. The patient was hemodynamically stable and was found to have an elevated troponin with electrocardiogram showing no ischemic changes. Computed tomography of the coronary arteries showed a left dominant system with dissection extending from the mid‐to‐distal left anterior descending (LAD) artery. The patient was subsequently discharged on medical therapy but returned 3 days later due to worsening chest pain. Electrocardiogram revealed inferior and anteroseptal ST segment changes with peak troponin of 14.9 ng/ml (reference range <0.80 ng/ml). Coronavirus disease 2019 (COVID‐19) nasopharyngeal swab was performed prior to urgent coronary angiogram. Coronary angiogram was performed with full personal protective equipment for respiratory and droplet precautions due to pending COVID‐19 testing results. Angiogram revealed spontaneous coronary artery dissection (SCAD) extending from the ostium of the LAD to the distal vessel. COVID‐19 testing returned positive while in intensive care unit. The patient was not a percutaneous coronary intervention candidate due to the extent of the dissection and was not a surgical candidate due to a lack of graftable target and medical management was continued. To our knowledge, this case is the first in which SCAD has been reported in the LAD in a patient with COVID‐19 with no other symptoms of respiratory illness or symptoms classically associated with the novel coronavirus. SCAD should be considered on the differential as one of the various cardiac manifestations of COVID‐19 infection.
Keywords: catheterization diagnostic, coronary angiography, coronary artery disease, coronary dissection
1. INTRODUCTION
Novel coronavirus disease 2019 (COVID‐19) as a result of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is responsible for the global pandemic that has infected over 1.5 million patients worldwide 1 with cardiac manifestations and injury in up to 20–28% of patients. 2 , 3 While the exact mechanism of cardiac injury in this population is unknown, the proposed etiology is thought to be due to changes in myocardial demand as a result of infection leading to an ischemic cascade and increased inflammatory markers that predispose patients to plaque instability and subsequent rupture. 4 Patients with cardiovascular injury have a fourfold increased risk of mortality compared to those without cardiovascular disease. 3
Coronary disease and myocardial infarction have been noted to occur concomitantly in patients with influenza and respiratory illnesses on both presentation and in autopsy studies. 5 , 6 COVID‐19 has been documented as a cause of heart failure, acute coronary syndromes, myocardial infarction, and myocarditis leading to life‐threatening arrhythmias. 7 Due to risk of transmission in both symptomatic and asymptomatic patients, protocols have been rapidly instituted in order to protect patients and healthcare workers to prevent further spread of disease. 8
We present a novel case of spontaneous coronary artery dissection (SCAD) in the left anterior descending (LAD) artery in a patient who was COVID‐19 positive with no other symptoms of SARS‐CoV‐2 to help illustrate the wide range of cardiac manifestations that patients can present with and for clinicians to keep the novel coronavirus in their differential as the cause of new cardiac complications.
2. CASE
A 48‐year‐old woman with a past medical history of migraines and hyperlipidemia presented to the emergency room due to a 1‐day history of severe chest pain that awoke her from sleep. She rated the pain as 9 out of 10 severity retrosternal tightness with radiation to her neck and bilateral arms. She was given aspirin 324 mg along with sublingual nitroglycerin for pain control with improvement in her symptoms. As recently as 1 day prior, she was exercising with no issues and had a functional capacity that exceeded 8 metabolic equivalents. She was afebrile without respiratory complaint.
Initial troponin I was <0.01 with subsequent troponin I of 0.5 ng/ml (reference range <0.80 ng/ml) with an electrocardiogram that did not show any acute ST changes or signs of ischemia. Vital signs remained stable and the patient was started on a heparin infusion and remained chest pain free, with troponin I peak of 1.41 ng/ml. Computed tomography coronary angiogram revealed a left dominant system. The LAD artery showed dissection from the mid‐to‐distal LAD (Figure 1). Transthoracic echocardiogram showed left ventricular ejection fraction of 45–50% and akinesis of the distal anteroseptal and apical segments.
FIGURE 1.
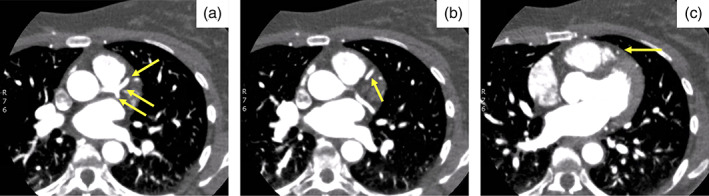
Coronary computed tomography showing dissection of the left anterior descending (LAD) artery. (a) Patent left main coronary artery, proximal LAD, and left circumflex arteries (arrows). (b) Patent proximal to mid LAD artery (arrow). (c) Spontaneous coronary artery dissection of the mid LAD artery (arrow)
The patient was diagnosed with SCAD and started on medical management including aspirin 81 mg daily and clopidogrel 75 mg daily. Prior to discharge, the patient underwent renal ultrasound that showed no evidence of fibromuscular dysplasia.
Three days postdischarge, the patient had recurrence of severe substernal chest pain rated at 9 out of 10 on pain scale. She was reloaded with full dose Aspirin and symptoms of pain resolved after administration of sublingual nitroglycerin and intravenous fentanyl. Electrocardiogram subsequently revealed submillimeter ST elevations in leads III and aVF, and biphasic changes in V3–V5 (Figure 2) with initial troponin of 0.11 ng/ml. The patient remained hemodynamically stable and was saturating well on room air. She was again afebrile and without respiratory complaint. Due to likely need for coronary angiogram to evaluate the extent of dissection and possible progression, a nasopharyngeal swab for COVID‐19 was performed on admission. On hospital day 1, troponin I rose to 14.9 ng/ml and severe chest pain recurred prompting urgent coronary angiography.
FIGURE 2.
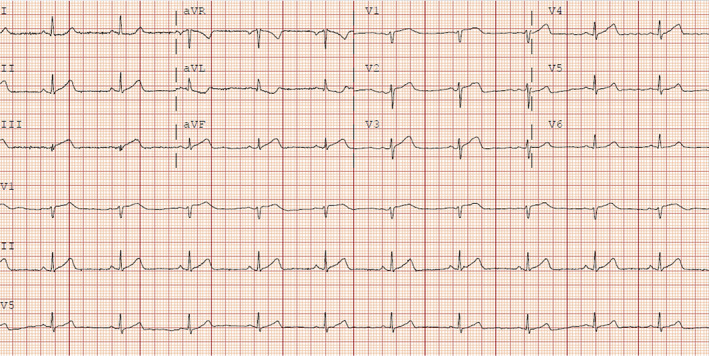
Electrocardiogram showing ST segment changes in the inferior and anteroseptal leads
As COVID‐19 testing was pending, full respiratory precautions and personal protective equipment (PPE) were utilized as a precautionary measure in the event of emergent intubation for airway protection or cardiopulmonary resuscitation (CPR). Access for angiography was via the right radial artery. A 5‐Fr JL 3.5 diagnostic catheter was attempted but was exchanged for a 5‐Fr EBU 3.0 guiding catheter to engage the left main coronary artery. Selective angiography of the left coronary system revealed SCAD. Tapered compression of 70% was noted at the mid through distal LAD with reconstitution at the apex. Delayed contrast washout was noted in the proximal LAD indicating the proximal extent of the dissection (Figure 3). The LAD was large and wrapped around the apex to supply the inferior septum. Left ventricular end‐diastolic pressure was normal (12 mmHg). The anatomy was deemed high risk for percutaneous coronary intervention due to the extent of SCAD. Chest pain had improved and given hemodynamic and electrical stability, medical therapy was continued.
FIGURE 3.
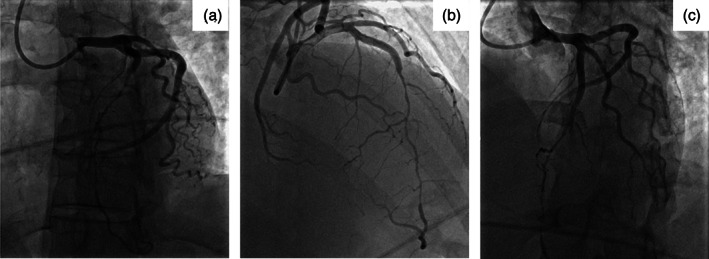
Coronary angiography. (a) Dissection of the left anterior descending (LAD) artery extending from the ostium to the distal vessel. (b) Wraparound LAD artery supplying the inferior septum. (c) Seventy percent compression of the LAD artery from the mid to distal vessel
Within 8 hr, COVID‐19 testing returned positive. The patient was evaluated by cardiothoracic surgery for possible surgical intervention but was declined as the LAD was not felt to be a graftable target. The patient later developed sustained polymorphic ventricular tachycardia for 45 s 2 days after coronary angiography likely secondary to ischemia, which spontaneously converted back to normal sinus rhythm. She was started on beta blocker and loaded with amiodarone with no recurrence of arrhythmia and continued on dual antiplatelet therapy for a minimum of 12 months for medical management of SCAD. Patient was discharged with a LifeVest (Zoll Medical, Chelmsford, MA), with plans for close follow‐up within 1 week.
3. DISCUSSION
Cardiac manifestations of COVID‐19 are various in etiology, ranging from acute coronary syndromes to heart failure with severe myocarditis causing ventricular arrhythmias that are often life threatening. Cardiac injury has also been associated with an increased risk of mortality in patients with SARS‐CoV‐2. We describe how a patient without respiratory symptoms with a pending COVID‐19 test was taken to the cardiac catheterization laboratory urgently for evaluation of SCAD and was later determined to be COVID‐19 positive.
Evaluation of patients presenting to the inpatient and outpatient settings during this global pandemic with cardiac chief complaints requires a thorough history and examination to evaluate for potential infection with SARS‐CoV‐2. The virus should be on the differential for all clinicians as a possible cause of cardiopulmonary complaints. Understanding the range of cardiac manifestations and how they can affect patients can help clinicians to further care for patients with potential COVID‐19 infection.
SCAD has not been widely reported as a presenting feature or complication in this patient population, yet our case highlights how SCAD can be the only presenting manifestation secondary to COVID‐19 and subsequent inflammatory response. Limiting transmission of the virus while protecting patients and members of healthcare team is a prime goal and cardiac catheterization laboratory protocols must be rapidly evolved to maintain high‐quality and safe cardiovascular care amidst the current pandemic. 9 , 10 Our case highlights the utility of universal COVID‐19 testing prior to catheterization procedures where feasible as well as the role of adequate PPE to protect team members in COVID‐19 unknown or pending cases given the risk of unplanned aerosol producing procedure such as intubation or CPR.
SCAD has previously been described in a patient with COVID‐19 in the right coronary artery with symptoms consistent with the respiratory manifestations of COVID‐19 upon presentation. 11 Our case is novel as it highlights how chest pain secondary to SCAD in the LAD may be the only presenting symptom in a patient with no other manifestations of the novel coronavirus. Understanding the various cardiac and noncardiac manifestations of COVID‐19 is important for clinicians to help deliver care to patients in the era of this global pandemic.
4. CONCLUSION
COVID‐19 causes a range of cardiovascular manifestations that should be considered in the care of this patient population with and without symptoms of respiratory illness attributable to SARS‐CoV‐2. Our case highlights how SCAD can be a presenting symptom of COVID‐19 infection in the absence of any other symptoms classically associated with the novel coronavirus and the rationale for universal COVID‐19 testing prior to catheterization laboratory procedures with adequate PPE in emergent cases where COVID status is frequently unknown. Understanding the various presentations of this virus and subsequent strategies of treatment is an important step to helping clinicians treat patients during the global pandemic.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Kumar K, Vogt JC, Divanji PH, Cigarroa JE. Spontaneous coronary artery dissection of the left anterior descending artery in a patient with COVID‐19 infection. Catheter Cardiovasc Interv. 2021;97:E249–E252. 10.1002/ccd.28960
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) pandemic. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed April 11, 2020.
- 2. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 5. Madjid M, Miller C, Zarubaev V, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy‐confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J. 2007;28(10):1205‐1210. 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smeeth L, Thomas S, Hall A, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611‐2618. 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 7. Akhmerov A, Marbán E. COVID‐19 and the heart. Circ Res. 2020;75(18) 2352‐2371. 10.1161/circresaha.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driggin E, Madhayan M, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020. 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szerlip M, Anwarduddin S, Aronow H, et al. Considerations for cardiac Catherization laboratory procedures during the COVID‐19 pandemic. Perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) members and graduates. Catheter Cardiovasc Interv. 2020;96(3):586‐597. [DOI] [PubMed] [Google Scholar]
- 10. Mahmud E. The evolving pandemic of COVID‐19 and interventional cardiology. Catheter Cardiovasc Interv. 2020;96(3):507‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Courand P, Harbaoui B, Bonnet M, Lantelme P. Spontaneous coronary artery dissection in a patient with COVID‐19. JACC Cardiovasc Interv. 2020. 10.1016/j.jcin.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


