Abstract
Background
Outpatient telemedicine consultations are being adopted to triage patients for head and neck cancer. However, there is currently no established structure to frame this consultation.
Methods
For suspected referrals with cancer, we adapted the Head and Neck Cancer Risk Calculator (HaNC‐RC)‐V.2, generated from 10 244 referrals with the following diagnostic efficacy metrics: 85% sensitivity, 98.6% negative predictive value, and area under the curve of 0.89. For follow‐up patients, a symptom inventory generated from 5123 follow‐up consultations was used. A customized Excel Data Tool was created, trialed across professional groups and made freely available for download at www.entintegrate.co.uk/entuk2wwtt, alongside a user guide, protocol, and registration link for the project. Stakeholder support was obtained from national bodies.
Results
No remote consultations were refused by patients. Preliminary data from 511 triaging episodes at 13 centers show that 77.1% of patients were discharged directly or have had their appointments deferred.
Discussion
Significant reduction in footfall can be achieved using a structured triaging system. Further refinement of HaNC‐RC‐V.2 is feasible and the authors welcome international collaboration.
Keywords: COVID‐19, follow‐up, new referrals, outpatient consultation, triaging
1. INTRODUCTION
Travel has been identified as the single most important contributor to the spread of the coronavirus disease 2019 (COVID‐19) pandemic. Reduction in the transmission of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has a direct link with the introduction of travel control measures. 1 The Wuhan shutdown delayed the occurrence of the first case of SARS‐CoV‐2 in other cities by 2.91 days (95% confidence interval: 2.54‐3.29 days), an intervention that benefited >130 cities in mainland China, covering more than half its geographic area. 24 Social distancing measures are also a key component of the control strategies during pandemics and form one of the most effective techniques in reducing the number of infections. It is also vital that social distancing and travel restrictions are not lifted prematurely while there is a pool of susceptible hosts in the population, as this will lead to an increase in the number of infections. 2 When applied to the health care sector these measures should aim to reduce hospital attendance by triaging out low risk patients both to protect clinicians and hospitalized patients, while still allowing timely investigations on those deemed to be at higher risk.
Interventions, such as outpatient telemedicine consultations, can reduce footfall in hospitals, thereby promoting adherence to social distancing policies. 3 These measures are especially relevant in the cohort of patients with head and neck diseases as the nose and nasopharynx have been shown to be reservoirs for high concentrations of the SARS‐CoV‐2. 4 Reduction of upper aerodigestive tract interventions, including outpatient examinations, is important as many are considered to be aerosol generating procedures. 5 In addition, SARS‐CoV‐2 remains viable in aerosols with a median half‐life of 1.1 hours, 6 potentially making the examination room a source of infection.
The National Health Service guidance for managing referrals with cancer during the COVID‐19 pandemic recommends a telephone triage to minimize interactions and appointments with health services and stream patients for investigations where appropriate. 7 In addition, a telephone appointment with a specialist clinician is accepted as a first appointment for the purposes of recording cancer waiting times for new referrals. As telephone triage is a relatively novel intervention for suspected head and neck cancer (HNC), there is currently no established structure to frame this consultation.
The aim of this study is to demonstrate a rapid implementation of an evidence‐based, structured, remote triaging system for assessment of suspected referrals and patients with cancer who are on regular follow‐up after treatment for HNC in the United Kingdom (UK).
2. RATIONALE
2.1. New referrals
A substantial part of outpatient care in head and neck surgical oncology work involves assessment of patients referred in with symptoms suspicious of HNC. Apart from a few malignancies (basal cell carcinomas, low‐grade salivary gland tumors and well‐differentiated thyroid cancer in young patients), most HNCs progress within a matter of months, and a delay in management can have an adverse effect on the prognosis. In 2000, the Department of Health in the United Kingdom developed national guidelines for referral of suspected HNC from primary care, updated by the National Institute for Health and Care Excellence in 2005 and 2015. Systematic reviews have shown that the pooled detection rate of cancer in this population is between 8.8% 8 and 11.1%. 9 Thus, the vast majority of referrals may be safely triaged to a deferred assessment.
In 2016, our group used information from a cohort of 4715 patients referred in for suspected HNC to generate a 13‐symptom inventory which, when combined with age, could generate a personalized risk of cancer with a high diagnostic efficacy (area under the curve [AUC] 0.77). 10 Sensitivity analysis identified the 8% risk probability cutoff to offer optimal performance for the calculator. This risk calculator was subsequently validated in an external cohort of 1998 patients, where it performed well compared to the inception cohort, with an AUC of 0.81. 11
After further refinements using additional information to the symptom inventory in a new cohort (n = 3531), we published the Head and Neck Cancer Risk Calculator (HaNC‐RC)‐V.2, which demonstrated increased diagnostic efficacy with an AUC of 0.89, and a sensitivity of 85%. 12 The model performed optimally at a probability cut off of 7.1%, where the negative predictive value was 98.6%. Thus, when the symptom inventory is applied to a patient who has been referred for suspected cancer and the calculator indicates a <7.1% risk probability for cancer diagnosis, the chance of missing cancer if the patient is not seen by a face‐to‐face conventional consultation is 1.4%. In all these instances, a logistic regression model was used to define personalized probability risk for each patient. When a variety of machine learning algorithms were used on a similar cohort of 5082 patients referred for suspected HNC, logistic regression, the technique used to create the calculator, offered one of the highest true negative rates, an essential characteristic of a test to triage patients out during resource constrained times. 13
2.2. Qualitative assessment of triaging from primary care practitioners and hospital specialists
SARS‐CoV‐2 has already changed the face of primary care in England; the need to reduce face‐to‐face interactions means clinicians are embracing new technology that allows remote assessment including SMS images and video consultations.
Our previous qualitative work (P. T. Bradley et al, unpublished data) to gauge the views of hospital specialists and primary care practitioners has been supportive of a risk calculator. This work used a normalization process theory (which evaluates implementation and complex interventions) to explore physicians' attitudes to the introduction and use of a head and neck clinical cancer decision tool (HaNC‐RC‐v.1) in both primary care and hospital practice.
The views of 11 head and neck surgeons from the North East of England were elicited via face‐to‐face interviews between March 6, 2019 and July 9, 2019. The interviewees welcomed an evidence‐based symptom tool and opined that a robust tool offered additional confidence to decision making. Concerns about triaging in the hospital setting were expressed as adequate information may not be available in the referral form; however, a structured triaging system such as the one proposed in this work directly addresses this concern. Primary care practitioners' views (12 general practitioners in the North East were interviewed face to face between June 14, 2019 and December 5, 2019) discussing the head and neck cancer risk calculator (version 1) as a means to drive more confidence in the triage of patients to suspected cancer clinics was met with enthusiasm. With endorsement from secondary care and careful integration into the pathway, it was felt that this approach could be an asset to both primary and secondary care (P. T. Bradley et al, unpublished data).
2.3. Follow‐up patients who have been treated for HNC
Change in patient symptoms during follow‐up is the most frequent indication of recurrent disease and must be regarded seriously, even if clinical examination reveals no abnormalities. 14
INTEGRATE, the UK Trainee Research Collaborative Network, performed a UK national audit of patients who underwent 5123 follow‐up consultations after treatment for HNC in 89 hospitals across the UK. 15 Residual or recurrent disease rates were 57% at 2 years, 32% between 2 and 5 years, and 11% post‐5 years follow‐up appointments expedited by either the patient or the clinician due to clinical concern correlated significantly with the presence of residual or recurrent disease, or a second primary tumor (P = .0001). The pick‐up rate was 35% in expedited appointments compared to 5.2% in planned follow‐ups. Of the expedited appointments, 63% were initiated by patients vs 37% by clinicians (Table 1).
TABLE 1.
New symptoms and their positive predictive values (PPV)
| New symptoms | PPV |
|---|---|
| Difficulty breathing | 16.2 |
| Tiredness | 12.9 |
| Pain in mouth/throat | 10.4 |
| Pain in neck/shoulder | 9.2 |
| Difficulty speaking | 8.4 |
| Difficulty swallowing | 7.9 |
| Bleeding | 7.3 |
| Dry mouth | 3.4 |
Parallels exist in other cancer sites where remote follow‐up is performed based on patent symptomatology and blood markers. Qaderi et al 16 optimized an innovative electronic medical record application for patients with colorectal cancer. Patients can review their appointments and test results, symptoms are monitored using online questionnaires; the long‐term results are awaited. However, in these resource constrained times, rapid innovation and dissemination of new care models are needed, which, with careful data collection and robust governance, can define new standards without causing patient harm.
3. METHODS
A focus group of five senior clinicians agreed that structured remote assessment could be performed, for new referrals, using the latest iteration of the risk calculator. It was agreed that those who were at high risk should be triaged for further assessment and the low‐risk referrals undergo a deferred assessment until later in the pandemic or when capacity became available. It was also considered appropriate for follow‐up patients to be asked if they had developed any new and specific symptoms since their previous consultation, allowing data from the 2018 audit to risk stratify this group to inform decisions regarding future management.
The project looked to collect data on clinician choice and patient preference and did not mandate treatment according to set protocols. Accordingly, the project was deemed to be a service evaluation and did not constitute research (http://www.hra-decisiontools.org.uk/research/).
The project was developed in collaboration with INTEGRATE. Through lending its support, and using its network to promote the project, all UK head and neck cancer centers were approached to consider participation. Further advertising was delivered through emails from ENT UK and the Association of Otolaryngologists in Training (www.aotent.org).
Designing the project in this way presented a number of challenges with the data collection strategy.
A need for immediate feedback to the clinician triaging the patient, and so the data must be entered directly into a computer interface.
A need for a complete data set to give valid results, again mandating a computer interface for data validation.
A need for follow‐up data to be collected at 6 months to see if patients subsequently develop cancer, and so patient identifiable data must be used in some capacity.
A need for rapid deployment of the project to ensure the newly implemented service to appropriately evaluated, and so formal applications for sponsorship or ethics may be too slow to achieve.
However, to comply with data governance regulations, patient identifiable information should not leave the institution and, if these were to, it must be handled with appropriate standards and practices to maintain confidentiality. Furthermore, formal consent may be required from the patient for their data to be used in this way.
The solution developed was a customized Excel Data Tool (Microsoft Excel for Mac. Redmond, Washington: Microsoft Corporation; 2018). This was made freely available for download at www.entintegrate.co.uk, alongside a user guide, protocol, and registration link for the project. Dates and hospital numbers may be entered into the Spreadsheet, which is stored on the hospital computer system, in line with local data governance regulations for handling patient identifiable data. Data entry is mostly limited to drop down lists and the decision aid only provides information if all required fields are completed. Clinician preference, patient choice and the immediate triage outcome can be recorded. Subsequently, the patient ID can be used to obtain the cancer status at 6 months follow‐up to complete the data set.
At this point, the spreadsheet can be anonymized by removing the site, the date of triage (if recorded), and the patient ID. Anonymous data only are then submitted to the project management team who then apply sequential study IDs for further analysis. Using these methods, the data held by the project management team have high levels of data completeness but are not traceable back to any individual center or individual patient.
Figure 1 summarizes the remote triaging process in a flowchart for new referrals and follow‐up patients, along with a recommended script to advise the patient on the outcome of the triaging process.
FIGURE 1.
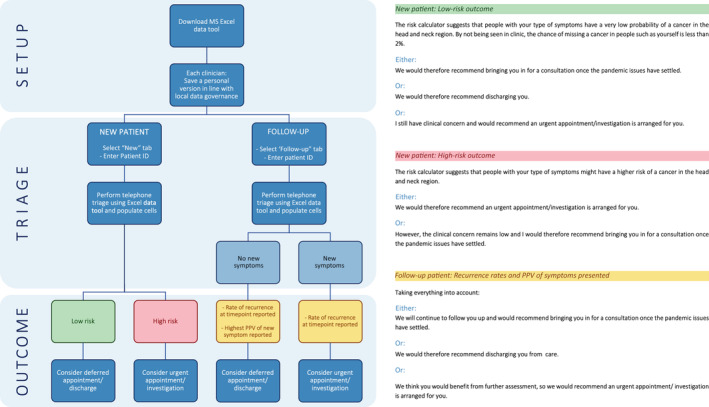
Flow chart demonstrating the remote triaging process for new referrals and follow‐up patients, along with a recommended script to advise the patient on the outcome of the triaging process [Color figure can be viewed at wileyonlinelibrary.com]
We intend for the service evaluation to continue for as long as telephone triage is being utilized in this patient group. It is anticipated that the period of disruption/telephone triage may last for at least 6 months. The outcome of presence/absence of cancer diagnosis at a minimum of 6 months will be treated as gold standard, and measures of diagnostic efficacy of the remote triaging system will be analyzed, including a descriptive analysis. This will allow external validation of the HaNC‐RC‐V.2 in a pan‐UK or even a global suspected referral population with head and neck cancer. Moreover, a detailed assessment of the false negatives will be carried out to investigate the potential scope for further refinement of the calculator.
4. RESULTS
None of the teleconsultations were refused by patients although some (<1%) expressed some dissatisfaction at the arrangements. The overwhelming majority understood the reasons for the remote consultation. Preliminary data were available from 511 triaging encounters from 13 centers; these show that 77.1% of patients were discharged directly or have had their appointments deferred until a later date; the remaining 22.9% were triaged to urgent investigations and/or face‐to‐face consultations. At time of submission, the project page at www.entintegrate.co.uk/entuk2wwtt had been accessed over 1800 times.
5. DISCUSSION
The UK sees an average of 100 000 suspected referrals with HNC per year, with a cancer pickup rate of less than 10%. Our preliminary results indicate that >80% of referrals can be triaged out through teleconsultation.
Risk calculators are available for several solid malignancies: these include bladder, brain, breast, colorectal, kidney, lung, esophagus, ovary, pancreas, prostate, and uterus. Some have been externally validated. Unlike other cancers where risk calculators need blood tests and radiology to be performed, the HaNC‐RC‐V.2 for HNC was generated solely from patient symptomatology and demographics, rendering it suitable for the purpose of teleconsultation and remote triaging. The HaNC‐RC‐V.2 has a higher AUC than most of the risk calculators published for other cancers. 17 As our model was based on recording of symptoms in the secondary care by specialist, it is feasible that the detailed recording of the symptoms during the consultation has helped to achieve higher predictive power than primary care derived models.
The use of telemedicine‐directed patient care during public health emergencies is well described. However, the use of digital technology in the COVID‐19 pandemic across the globe, especially in contact tracing and testing, has been unprecedented. Concurrently, the use of digital solutions for health care delivery has been accelerating since the pandemic began. In England, primary care has embraced telemedicine and deploys a new digital first pathway to manage and stream care, with over 90% of consultations being remote. Other examples include a central dashboard to manage bed availability within hospital settings and use of personalized online screening. A structured approach to remote triaging and generation of a personalized risk probability will allow clinical assessment with a consistency that otherwise cannot be delivered in this environment.
In the emergency setting, an important strategy for health care surge control during disaster management is forward triage. In the COVID‐19 pandemic model, this involves remote assessment of patients before patients arrive to the emergency care services in the hospital, either in a location proximate to the hospital or via teleconsultation. Emergencies that are unrelated to COVID‐19 are triaged to the emergency department while patients showing signs of the virus are separated to prevent transmission. The structured remote use of the risk calculator in the pandemic time is akin to the forward triage, where following appropriate assessment, to prevent a surge when health care is rationed, patients considered to be high risk are directed to the specialist for further assessment. Ideally the tool would be used in primary care but for expediency in the current medical crisis and given the fact it is a secondary care‐derived model it was felt best placed there for triage.
Remote consultations will be a prominent part of the outpatient clinical practice for the near future. Telehealth services have been promoted actively during the COVID‐19 pandemic setting for initial screening of symptomatic patients or those referred for medical care in other specialties and by more than 50 health systems in the United States. 18 Structured remote assessment of sick patients has been described in the pandemic era. 19 Research indicates that with appropriate structure and guidance, a teleconsultation model can be successful. 20
It is very likely that patients will be willing to engage with teleconsultation when face‐to‐face access to health care is restricted. Using internet search volume data from Google Trends, Hong et al 21 showed that the U.S. population's interest in telehealth increased as the number of COVID‐19 cases increased, with a strong correlation between population interest and COVID‐19 cases reported (r = .948, P < .001).
Medical decision making is cognitive, especially as experience accumulates. However, in the early phases of training, when conventional assessment cannot be performed and where there is less opportunity for supervision, a structured telemedical approach, backed up by robust algorithmic approach that has been generated and validated from the population at risk, will be of significant help in reducing anxiety among the clinical team and provider organization. As always, we would recommend experience and clinical judgment supersede the output on the screen. To the best of our knowledge, this is the first remote‐structured assessment tool that has been robustly generated, validated, and rapidly implemented for use in the HNC setting.
5.1. Stakeholder and international collaboration
This structured triaging system has been endorsed by ENT UK, 22 the official body representing British Otolaryngologists—Head and Neck Surgeons and the British Association of Head and Neck Oncologists. 23 The authors would welcome international collaboration, and prospective centers are invited to visit the project page at www.entintegrate.co.uk/entuk2wwtt.
It is anticipated that the COVID‐19 pandemic will influence clinical care for several years to come. The data generated from a real‐world triaging such as this, when collected under robust data governance and oversight, analyzed and refined to reduce patient harm even further, can influence health care provision for years to come.
Paleri V, Hardman J, Tikka T, Bradley P, Pracy P, Kerawala C. Rapid implementation of an evidence‐based remote triaging system for assessment of suspected referrals and patients with head and neck cancer on follow‐up after treatment during the COVID‐19 pandemic: Model for international collaboration. Head & Neck. 2020;42:1674–1680. 10.1002/hed.26219
REFERENCES
- 1. Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID‐19: a mathematical modelling study. Lancet Infect Dis. 2020;20:553‐558. 10.1016/S1473-3099(20)30144-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prem K, Liu Y, Russell TW, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID‐19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261–70. 10.1016/S2468-2667(20)30073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebrahim SH, Ahmed QA, Gozzer E, Schlagenhauf P, Memish ZA. Covid‐19 and community mitigation strategies in a pandemic. BMJ. 2020;368:m1066. 10.1136/bmj.m1066 [DOI] [PubMed] [Google Scholar]
- 4. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Public Health England . COVID‐19 Personal Protective Equipment (PPE). London: Public Health England; 2020. [Google Scholar]
- 6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New Engl J Med. 2020;382:1564‐1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NHS England and NHS Improvement . Cancer Alliance Information on Managing Cancer Referrals. London: NHS England and NHS Improvement; 2020. [Google Scholar]
- 8. Langton S, Siau D, Bankhead C. Two‐week rule in head and neck cancer 2000‐14: a systematic review. Br J Oral Maxillofac Surg. 2016;54:120‐131. 10.1016/j.bjoms.2015.09.041 [DOI] [PubMed] [Google Scholar]
- 9. Kumar RDM, Mehanna H, Paleri V. Efficacy of the two week wait referral system for head and neck cancer: a systematic review. Ann R Coll Surg Engl (Supp). 2012;94:102‐106. [Google Scholar]
- 10. Tikka T, Pracy P, Paleri V. Refining the head and neck cancer referral guidelines: a two‐centre analysis of 4715 referrals. Clin Otolaryngol. 2016;41:66‐75. 10.1111/coa.12597 [DOI] [PubMed] [Google Scholar]
- 11. Tikka T, Paleri V, MacKenzie K. External validation of a cancer risk prediction model for suspected head and neck cancer referrals. Clin Otolaryngol. 2018;43:714‐717. 10.1111/coa.13019 [DOI] [PubMed] [Google Scholar]
- 12. Tikka T, Kavanagh K, Lowit A, et al. Head and neck cancer risk calculator (HaNC‐RC)‐V.2. Adjustments and addition of symptoms and social history factors. Clin Otolaryngol. 2020;45:380‐388. 10.1111/coa.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moor JW, Paleri V, Edwards J. Patient classification of two‐week wait referrals for suspected head and neck cancer: a machine learning approach. J Laryngol Otol. 2019;133:875‐878. 10.1017/S0022215119001634:1-4 [DOI] [PubMed] [Google Scholar]
- 14. Simo R, Homer J, Clarke P, et al. Follow‐up after treatment for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S208‐S211. 10.1017/S0022215116000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardman J, Tikka T; on behalf of INTEGRATE (The National ENT Trainee Research Network). United Kingdom Head and Neck Cancer Surveillance study 2018. Paper presented at: British Association of Head and Neck Oncology 2019 Annual Scientific Meeting; London, England.
- 16. Qaderi SM, Vromen H, Dekker HM, Stommel MWJ, Bremers AJA, de Wilt JHW. Development and implementation of a remote follow‐up plan for colorectal cancer patients. Eur J Surg Oncol. 2020;46:429‐432. 10.1016/j.ejso.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 17. Usher‐Smith J, Emery J, Hamilton W, Griffin SJ, Walter FM. Risk prediction tools for cancer in primary care. Br J Cancer. 2015;113:1645‐1650. 10.1038/bjc.2015.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid‐19. N Engl J Med. 2020;382:1679‐1681. 10.1056/NEJMp2003539 [DOI] [PubMed] [Google Scholar]
- 19. Greenhalgh T, Koh GCH, Car J. Covid‐19: a remote assessment in primary care. BMJ. 2020;368:m1182. 10.1136/bmj.m1182 [DOI] [PubMed] [Google Scholar]
- 20. Seuren LM, Wherton J, Greenhalgh T, Cameron D, A'Court C, Shaw SE. Physical examinations via video for patients with heart failure: qualitative study using conversation analysis. J Med Internet Res. 2020;22:e16694. 10.2196/16694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong YR, Lawrence J, Williams D Jr, Mainous IA. Population‐level interest and telehealth capacity of US hospitals in response to COVID‐19: cross‐sectional analysis of Google Search and National Hospital Survey Data. JMIR Public Health Surveill. 2020;6:e18961. 10.2196/18961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ENT UK . ENT UK Guidelines for changes in ENT during COVID‐19 Pandemic. London: ENT UK; 2020. [Google Scholar]
- 23. British Association of Head and Neck Oncologists . ENTUK2WW Triage. London: British Association of Head and Neck Oncologists; 2020. [Google Scholar]
- 24. Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID‐19 epidemic in China. Science. 2020. 10.1126/science.abb6105 [DOI] [PMC free article] [PubMed] [Google Scholar]


