Changes in dietary behavior with increased adherence to a Mediterranean diet can determine a reduction of periodontopathogenic bacterial abundances in the saliva of overweight subjects with cardiometabolic risk due to an unhealthy lifestyle, without any change in individual energy intake, nutrient intake, and physical activity.
KEYWORDS: salivary microbiota, periodontitis, nutritional intervention, Mediterranean diet
ABSTRACT
The human oral cavity is a complex ecosystem, and the alterations in salivary microbial communities are associated with both oral and nonoral diseases. The Mediterranean diet (MD) is a healthy dietary pattern useful for both prevention and treatment of several diseases. To further explore the effects of the MD on human health, in this study, we investigated the changes in the salivary microbial communities in overweight/obese subjects after an individually tailored MD-based nutritional intervention. Healthy overweight and obese subjects were randomized between two intervention groups. The MD group (Med-D group) increased their MD adherence during 8 weeks of intervention while the control diet (control-D) group did not change their dietary habits. The salivary microbiota was assessed at baseline and after 4 and 8 weeks of intervention. Despite no observed changes in the overall salivary microbiota composition, we found a significant decrease in the relative abundances of species-level operational taxonomic units annotated as Porphyromonas gingivalis, Prevotella intermedia, and Treponema denticola in the Med-D group compared to that in the control-D group after 8 weeks of intervention, which are known to be associated with periodontal disease. Such variations were significantly linked to dietary variables such as MD adherence rates and intakes of animal versus vegetable proteins. In addition, increased levels of Streptococcus cristatus were observed in the Med-D group, which has been reported as an antagonistic taxon inhibiting P. gingivalis gene expression. Our findings suggest that an MD-based nutritional intervention may be implicated in reducing periodontal bacteria, and an MD may be a dietary strategy supportive of oral homeostasis.
IMPORTANCE Changes in dietary behavior with increased adherence to a Mediterranean diet can determine a reduction of periodontopathogenic bacterial abundances in the saliva of overweight subjects with cardiometabolic risk due to an unhealthy lifestyle, without any change in individual energy intake, nutrient intake, and physical activity.
INTRODUCTION
The human oral cavity is a well-studied ecosystem, harboring a complex community of microorganisms. More than 700 bacterial species inhabit the site, making up the oral microbiota (1), and it is considered the second most complex symbiont microbiota in the human body after the gut (2). Different individuals display a vast genetic variation in the oral microbial ecology. This dynamic ecosystem is influenced by both biological host parameters, including age (3), health status (4), body mass index (BMI) (5), and genetic predisposition (6), and local environmental factors such as diet (7), geographical environment (8), antibiotic consumption (9), and smoking (10). In addition, the oral microbiota composition varies among different oral sites (11); therefore, a healthy composition of the oral microbiota cannot be easily defined (12). Surprisingly, despite remarkable inter- and intraindividual variability, the microbial composition of the oral cavity is stable over time, and saliva exhibits high evenness in terms of microbial diversity (13, 14).
Several studies in recent years documented that alterations in salivary microbial communities are associated with both oral and nonoral disease (15), and specific components of the salivary microbiota were proposed as predictive biomarkers for different types of pathologies (16–18). Recently, the oral microbiota dysfunction has been associated with atherosclerosis and cardiovascular disorder: oral symbionts may indirectly elicit the immune dysregulation leading to the progressive inflammation associated with cardiovascular diseases (19). Diet is a fundamental factor affecting human health and the microbiome (20–22). However, studies linking diet to oral microbiota composition are still lacking. Indeed, in a cross-sectional observational study, we did not find microbial signatures in the salivary microbiota of individuals consuming an omnivorous, vegetarian, or vegan diet, while the salivary metabolome was better correlated with dietary habits (7). The Mediterranean diet (MD) is a healthy dietary pattern. High-level adherence to the MD is associated with reduced risk of the major chronic inflammatory diseases (23) and with a particular composition of gut microbiota and metabolome (24). Dietary interventions are the gold standard to establish causal variation in the human microbiota composition (25). Although several studies have been focused on the effect of dietary interventions on the gut microbiota, still little is known about the possible effects on the oral microbial ecology. To further explore the effects of the MD on human health, in this study, we investigated the global changes in the salivary microbial communities in overweight and obese subjects after an 8-week nutritional intervention with an MD.
RESULTS
Forty-nine subjects (29 [13 male and 16 female] in the MD group [Med-D group] and 20 [9 male and 11 female] in the control diet [control-D] group) from the study population described by Meslier and coworkers (26) successfully collected saliva samples throughout the nutritional intervention. The general characteristics of all participants over the study period, anthropometric data, and clinical variables measured in blood and urine samples along with diet variables are reported in Table S1 in the supplemental material. As expected with the protocol, which consisted of an isocaloric intervention, no significant differences in BMI, body weight, waist and hip circumferences, systolic and diastolic blood pressure levels, clinical parameters, or any intermediate markers of metabolic disease were observed between the control-D and Med-D groups either at baseline and over the study period (Table S1). The findings observed for this subgroup were consistent with those for the entire study population (26). Indeed, successful compliance to the intervention was achieved, with a significant increase in the Italian Mediterranean index score (ItMedIndex) in the Med-D group compared to that in the control-D group after 4 and 8 weeks (P < 0.001) (Table S1). Accordingly, higher levels of consumption of fruits, vegetables, and whole-grain cereal products in the Med-D group were confirmed by a significant increase in dietary fiber and dietary plant/animal protein ratio intake throughout the intervention compared to that in the control-D group (P < 0.001) (Table S1). Moreover, a significant reduction in saturated fat intake and an increase in polyunsaturated fat intake were also observed (P < 0.001) (Table S1).
Effects of the dietary treatment on the overall salivary microbiota composition.
We analyzed the bacterial compositions of 145 saliva samples from overweight/obese subjects. The overall microbial diversity did not differ significantly between Med-D and control-D groups at baseline or after the dietary intervention. Likewise, the weighted and unweighted UniFrac distances were unchanged after the treatment, indicating that the microbial communities in both groups were similar. Accordingly, we did not observe any clustering of the subjects according to the dietary intervention or the group (Med-D or control-D), suggesting no remarkable differences in the overall microbiota composition after the dietary treatment (see Fig. S1). A core microbiota with 13 genus-level operational taxonomic units (OTUs) (Actinomyces, Atopobium, Rothia, Porphyromonas, Prevotella, Granulicatella, Gemella, Streptococcus, Moryella, Veillonella, Neisseria, Haemophilus, and Leptotrichia) shared by 99% of the samples was identified (see Fig. S2). These genera belonged to the phyla Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Fusobacteria. Moreover, these microbial taxa were not affected by the dietary intervention, although their relative abundances showed great interindividual variability. There was no significant difference in the average abundances of the core microbial genera between the two intervention groups, both at baseline and after the treatment with the MD or control diet for 4 or 8 weeks. In all the samples, OTUs assigned to Prevotella genus occurred with the highest relative abundance (22.6% ± 9.1%), followed by OTUs annotated as Streptococcus (16.5% ± 6.3%), Veillonella (10.4% ± 4.3%), Neisseria (8.9% ± 6.5%), Porphyromonas (6.2% ± 4.8%), Rothia (5.3% ± 4%), Actinomyces (4% ± 2.1%), Haemophilus (4% ± 4%), Granulicatella (2.7% ± 1.2%), Leptotrichia (2.4% ± 2.2%), and Gemella (2% ± 1.2%).
Microbial changes linked to the MD intervention.
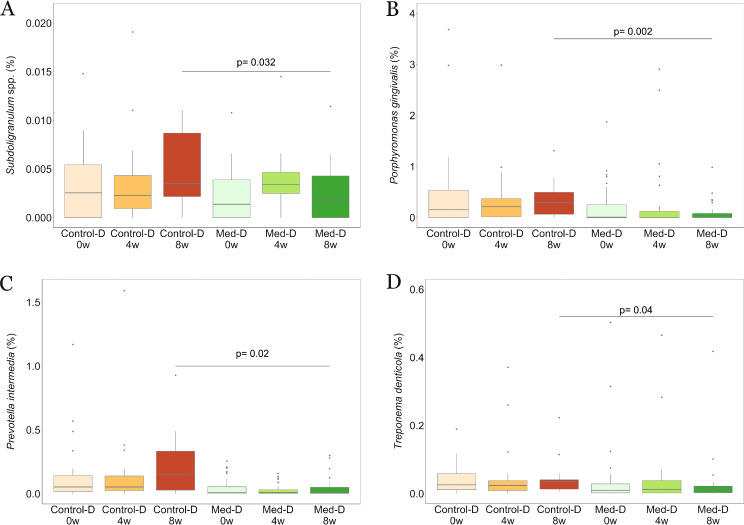
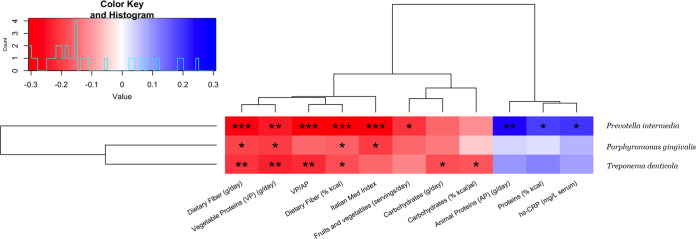
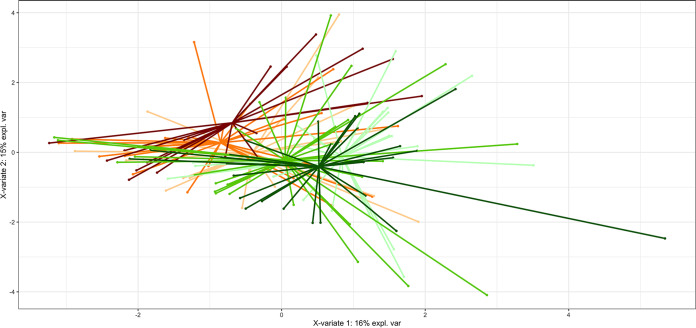
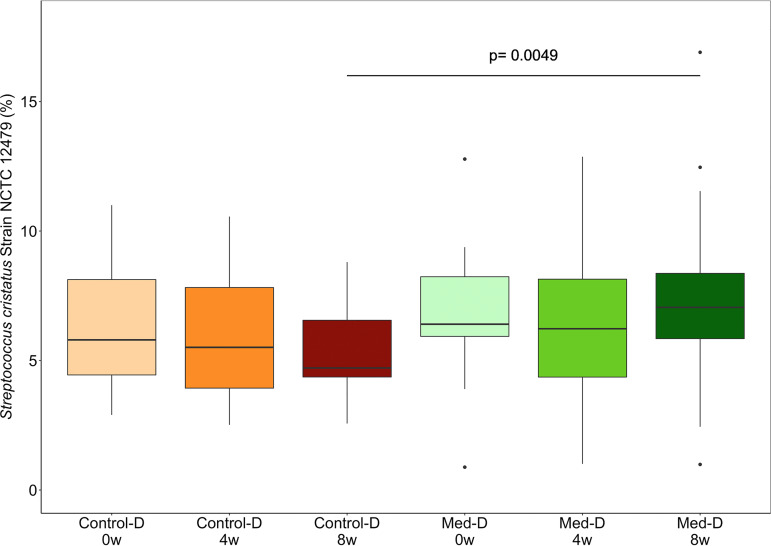
We found differences in the microbial community compositions between the two intervention groups, consisting of specific OTUs that varied in relative abundance upon dietary intervention. In particular, significant decreases in the relative abundances of the OTU assigned to genus Subdoligranulum (P < 0.05) and three OTUs annotated at the species level as Treponema denticola (P < 0.05), Porphyromonas gingivalis (P < 0.005), and Prevotella intermedia (P < 0.05) were observed after 8 weeks of treatment with the MD (Fig. 1). An additional phylogenetic analysis was performed including sequences of the V3-V4 amplicon regions for each of the annotated OTUs and by aligning these against the complete 16S rRNA reference sequences retrieved for all the species in the genera Treponema, Porphyromonas, and Prevotella. These analyses confirmed the taxonomical identification for each of these three taxa annotated at the species level (see Fig. S3 to S5). The relative abundances of such species OTUs were found to be significantly anticorrelated with the ItMedIndex and to dietary fiber and vegetable protein intake (Fig. 2). Interestingly, the OTU assigned to P. intermedia was positively associated with animal protein consumption and with the individual inflammatory status evaluated by serum C-reactive protein (hs-CRP) levels (Spearman’s ρ = 0.25 and 0.2, respectively). In addition, hs-CRP measures were found to be negatively correlated with the ItMedIndex and MD adherence (Spearman’s ρ = −0.21, P value = 0.012). Lastly, lower levels of OTUs annotated at the genus level as Streptococcus, Filifactor, and Lactobacillus were registered after both 4 and 8 weeks of dietary treatment with the MD, although such differences failed to reach significance. To explore the possible effects of the MD at a subgenus level, oligotyping of sequences from OTUs assigned to genera Prevotella and Streptococcus was carried out; these genera were chosen as the two most abundant. The oligotyping analysis led to 83 and 18 different oligotypes for Prevotella and Streptococcus, respectively. The diversity in oligotype compositions did not separate subjects belonging to different groups for both genera. Indeed, the dietary intervention did not affect the subgenus diversity, since all oligotypes were closely related in a hierarchical clustering analysis based on the distance matrix as reported for the OTU assigned to the genus Streptococcus (Fig. 3). However, Streptococcus oligotype S3, identified as Streptococcus cristatus after comparison with the human oral microbiome database (HOMD) 16S rRNA RefSeq database, showed a significant increase in its relative abundance within the Med-D group compared to that in the control-D group after 8 weeks of dietary treatment, as shown in Fig. 4 (P < 0.05).
FIG 1.
Box plots showing the relative abundances of Subdoligranulum spp. (A), P. gingivalis (B), P. intermedia (C), and T. denticola (D) from saliva samples of the study population. Subjects belonging to different categories were colored according to diet and time points: Med-D subjects at baseline (light green) and after 4 (green) and 8 (dark green) weeks of intervention and control-D subjects at baseline (light orange) and after 4 (orange) and 8 (dark orange) weeks of intervention. The significance was tested by applying unpaired Wilcoxon rank-sum tests comparing Med-D and control-D subjects. 0w, baseline; 4w, 4 weeks; 8w, 8 weeks of nutritional intervention.
FIG 2.
Correlations of periodontopathogenic bacteria with dietary and clinical variables. Heat map showing hierarchical Ward-linkage clustering of periodontopathogenic bacteria based on Spearman’s correlations with dietary variables and clinical parameters. The color scale represents the scaled version of Spearman’s ρ coefficients, with red indicating negative and blue indicating positive correlations. VP/AP, vegetable/animal protein ratio. Adjustments were performed using the Benjamini-Hochberg procedure, and Spearman’s ρ values were filtered by keeping correlations with at least one false discovery rate (FDR) of ≤0.05. *, FDR < 0.05; **, FDR < 0.01; ***, FDR < 0.001.
FIG 3.
Partial least-squares discriminant analysis (PLS-DA) model based on Streptococcus subgenus diversity matrix showing the clustering of different subjects according to categories. Subjects belonging to different categories were colored according to diet and time points (see the legend to Fig. 1 for details).
FIG 4.
Box plot showing the relative abundances of Streptococcus cristatus oligotypes in the categories analyzed in this study. Subjects belonging to different categories were colored according to diet and time points (see the legend to Fig. 1 for details).
DISCUSSION
The human oral microbiota is a complex ecosystem, and its homeostasis is important to contrast opportunistic pathogens and dysbiosis, potentially resulting in both oral inflammation and systemic infection (27). Here, we described the effect of the MD on the oral microbial ecology in overweight and obese subjects at cardiovascular risk due to an unhealthy lifestyle, comprising an unhealthy diet and a low level of physical activity (26). The overweight condition is a worldwide public health syndrome, associated with systemic low-grade inflammation and correlated with gut microbiota dysbiosis (28, 29). It has been reported that overweight status is positively associated with an increased risk of oral chronic inflammatory diseases such as periodontitis (30, 31), which in turn is caused by oral dysbiosis and facilitates oral colonization by pathogens (27). On the other hand, possible mechanisms by which oral bacteria could affect body weight and contribute to the overweight condition have been proposed (32) along with microbiological and biochemical differences distinguishing saliva samples from obese and from normal-weight individuals (5).
The detected taxa in 99% of saliva samples largely coincided with those reported in previous studies. We previously observed that the salivary microbiota was not significantly linked to specific dietary habits, although a core microbiota was identified, which included 6 of the 13 core genera identified here (7). In line with our results, other core members (Veillonella, Gemella, Actinomyces, and Rothia) coincided with those found in saliva samples in other studies (11). The shared genera seem to be part of common oral commensals, as the previous studies were performed on healthy cohorts. In addition, the overall microbiota composition was shown to be resistant to external perturbations as well as dietary intervention. This finding is consistent with other researches showing that the human salivary microbiome is stable in adulthood as a result of lifestyle and environmental factors (33).
Despite interindividual variability and host and environmental effects, an understanding of the oral microbiome could have a potential role for the prevention and management of diseases (15). We detected the effects of diet on OTUs well known to be associated with both oral and systemic diseases. Interestingly, after 8 weeks, there was a significant decrease in the relative abundance of OTUs annotated as Prevotella intermedia, Porphyromonas gingivalis, and Treponema denticola in the Med-D group compared to that in control-D subjects. These microorganisms were classified as periodontopathogenic bacteria, and the last two are members of the “red bacterial complex” (34). Although periodontitis is mainly driven by subgingival biofilm dysbiosis (35), the salivary microbiota was found to be a promising target for the evaluation of subgingival plaque-derived bacteria reflecting the periodontal health condition (36). Moreover, previous studies documented that dietary components from long-term dietary patterns or a change of diet can perturb salivary microbiota (37, 38), suggesting that microbial shifts in the saliva composition due to nutritional interventions might be valuable information in order to plan strategies of disease prevention. Due to their virulence factors, such oral pathogens can be responsible for periodontitis disease and following oral tissue destruction, subgingival pathogen colonization, and host defense immunomodulation (39). These periodontal pathogens have a proteolytic pool of enzymes involved in adhesion and nutrition phases. In addition, they are nonsaccharolytic microorganisms, and they require peptides and amino acids to grow and adhere to the gums (40).
The MD is a vegetarian-oriented dietary pattern providing many polyphenols with well-known antibacterial activity (41–43). In this respect, some in vitro studies showed that dietary polyphenols such as theaflavins, typical of tea, markedly inhibit the proteinase activities of P. gingivalis in a dose-dependent manner (44), thus limiting the adhesion capacity of these species in the oral cavity. Similarly, catechins and ellagic acids other than those in tea, coffee, and pomegranates, such as those contained in many fruits and vegetables as well as nuts typical of an MD, have been shown to inhibit the growth of Prevotella intermedia (45–47). Likewise, cranberry juice constituents were previously shown to contrast the proteolytic activity of T. denticola (48), while pomegranate juice inhibited the biofilm formation of the same bacterium (49). Besides polyphenols, other phytochemicals, including vitamin C (ascorbic acid), vitamin E (α-tocopherol), vitamin A, β-carotene, and coenzyme Q-10, and minerals provided by fruits, vegetables, nuts, and whole grains in the MD are effective for maintaining periodontal homeostasis (50–53). Indeed, Skoczek-Rubińska and colleagues (54) found that the consumption of at least 5 servings of fruits and vegetables per day may prevent the progression of periodontal diseases. Accordingly, in our intervention, species-level OTUs annotated as periodontopathogenic bacteria were found to be anticorrelated with dietary fiber intake and to the Mediterranean diet adherence score.
Periodontal diseases have also been associated with high levels of C-reactive protein (55, 56), to which Prevotella intermedia was significantly correlated in our study. Moreover, the levels of adherence to the Mediterranean diet were found to be negatively correlated with hs-CRP measures, despite the fact that such a marker was not significantly different between the two intervention groups. However, since the chronic oral infection of periodontitis may be a risk factor for systemic pathologies (15, 18, 57), the effect exerted by the adoption of a recommended nutritional pattern such as the MD might be relevant in reducing both periodontal bacteria and risk factors in the oral cavity, possibly improving oral health. In addition, we observed a significant increase in the Streptococcus cristatus oligotype in the Med-D group compared to that in the control-D group. Streptococcus cristatus was found to inhibit virulence gene expression in P. gingivalis with a direct interaction. Hence, the observed decrease in the relative abundance of the OTU assigned to P. gingivalis species in Med-D subjects might be attributed to an antagonistic presence of S. cristatus (58).
A significant decrease of a genus-level OTU classified as Subdoligranulum (P < 0.05) was observed after 8 weeks of treatment. Subdoligranulum species are correlated with inflammation in type 1 diabetes, although this was observed in fecal samples (59). Recent evidences suggest the hypothesis that the oral microbiome is linked to the gut microbiome. Indeed, metabolic disorders could be enhanced by swallowed oral pathogens, as shown in mouse models (60), and gut microbiota alterations under diabetic condition could cause a pathogenic shift in the oral microbiome leading to oral dysbiosis (61).
In conclusion, our findings suggest the MD modulates the salivary levels of the red bacterial complex, which is relevant for oral health. Specifically designed studies will be needed to further support the use of a Mediterranean diet as a dietary strategy to prevent periodontitis and to shed light on the implicated pathophysiological mechanisms.
MATERIALS AND METHODS
Study subjects.
The volunteers were recruited at the University of Naples Federico II in the frame of the diet-induced arrangement of the gut microbiome for the improvement of cardiometabolic health (DINAMIC) project within the European Joint Programming Initiative: a Healthy Diet for a Healthy Life (JPI HDHL)–Joint Action Intestinal Microbiomics. The trial is registered at ClinicalTrials.gov (number NCT03071718). Detailed inclusion and exclusion criteria were reported by Meslier et al. (26). Briefly, we enrolled healthy subjects aged from 20 to 60 years, with body mass indexes (BMIs) ranging from 28 to 35 kg/m2, a sedentary lifestyle, and a daily intake of fruit, vegetables, or whole grains of fewer than 3 portions/day. Contrarily, we excluded subjects with food-related allergies or intolerances (coeliac disease or lactose intolerance), any gastrointestinal disease, chronic or metabolic diseases, blood triglycerides of >300 mg/dl and total cholesterol of >220 mg/dl, with hypertension, consuming pre- and/or probiotics, antibiotics and/or medicine up to 3 months before the enrollment, previous surgical intervention, or simultaneous participation in other interventional studies as well as pregnant or breastfeeding women. Subjects participating in the present study were also nonsmokers, declared they brushed their teeth regularly, and had not suffered from any active oral or dental disease that caused bleeding gums for at least 4 weeks. Dietary habits were collected by weighed-food diaries compiled by the participants before starting the protocol and every 2 weeks.
Dietary intervention and sample collection.
Forty-nine subjects were enrolled in this subgroup of participants who, additionally to the procedures described by Meslier et al. (26), agreed to collect saliva samples throughout the intervention period according to specific instructions. Participants had to be fasting and not consuming chewing gum or any beverage but water, and they were not allowed to brush their teeth and/or use antimicrobial mouth rinses (if in their habits) since the evening (8 to 10 h) before the collection. In addition, they had to report that they did not have bleeding gums on the visit day or in the previous 4 weeks. Enrolled subjects were randomly assigned to receive an individually tailored Mediterranean (Med-D; n = 29) or control (control-D; n = 20) diet for 8 weeks. The MD assigned for each subject was based on a Mediterranean diet model, but it was isocaloric and maintained the macronutrient composition of the habitual diet. Particularly, Med-D group subjects increased their intake of fruit, vegetables, legumes, nuts, fish, olive oil, and whole-grain products while they reduced meat and dairy products without any change in alcohol consumption. On the contrary, subjects in the control-D group continued with their habitual diet. The compliance was assessed with weekly food diaries. Adherence to the MD is reported as the Italian Mediterranean index score (ItMedIndex) (62). At the beginning of the intervention (baseline, 0w) and after 4 weeks (4w) and 8 weeks (8w), fasting subjects reached the clinic and, after a visit from a medical doctor guaranteeing they were in a healthy condition, they entered the visit flow. Before blood drawings, the subjects self-declaring that they did not have any oral issues and had not changed oral hygiene habits over the last 4 weeks, did not have gum bleedings, and had followed the specific instructions for saliva collection were allowed to collect a saliva sample. Two milliliters of whole unstimulated saliva was collected by each subject through direct and passive salivation into a preweighed 50-ml sterile Falcon tube (63). Once collected, the sample was immediately placed on ice to minimize degradation of components until aliquoting in prelabeled sterile Eppendorf tubes (2 ml) and freezing at −80°C prior to DNA extraction.
DNA extraction and 16S rRNA gene sequencing.
Saliva samples were processed as previously described (7); aliquots of 2 ml were centrifuged (10,000 × g; 1 min), and the pellet was used for DNA extraction by using the QIAamp BiOstic Bacteremia DNA kit (Mo Bio Laboratories, Inc., Carlsbad, CA). The V3-V4 region of the 16S rRNA gene was amplified by using primers and PCR conditions recently described (64). Amplicon libraries were sequenced on a MiSeq platform, leading to 2 × 250-bp paired-end reads.
Bioinformatics and statistical analysis.
Demultiplexed forward and reverse reads were joined by using FLASH (65). Reads with a Phred score of <30 were trimmed by Prinseq (66) and those shorter than 250 bp were discarded. High-quality reads were analyzed by QIIME 1.9 (67), with a pipeline recently described (64). Statistical analysis and visualization were carried out using the R environment (https://www.r-project.org). The principal-component analysis (PCA) was assessed on log10-transformed operational taxonomic unit (OTU) tables by using dudi.pca function (library made4). To separate groups based on microbial profiles, a partial least-squares discriminant analysis (PLS-DA) was performed with the plsda function (library mixOmics). Statistical differences in differentially abundant taxa and dietary and clinical variables at specific time points between the Med-D and control-D groups were evaluated by nonparametric Wilcoxon signed-rank test. P values were corrected for multiple testing using the Benjamini-Hochberg procedure. To identify discriminant taxa avoiding incorrect identification, all picked OTUs found to be significantly different between groups were double checked against the human oral bacterial 16S rRNA gene sequences available at the human oral microbiome database (HOMD) platform (http://www.homd.org/). OTUs identified as periodontopathogenic bacterial species were further investigated to confirm their taxonomic assignment. Briefly, representative reads for each species-level OTU for Porphyromonas gingivalis, Prevotella intermedia, and Treponema denticola were sorted and aligned against a total of 23, 75, and 64 sequences of the genera Porphyromonas, Prevotella, and Treponema, respectively, downloaded from the eHOMD 16S rRNA reference gene sequences (V15.2) for phylogenetic analysis. The NGPhylogeny.fr tool (68) was used to provide the multiple-sequence alignment, the alignment curation, and the phylogenetic tree inference using FastTree. The cladograms were obtained using the Interactive Tree of Life (69). Pairwise Spearman’s rank correlations were used to test the correlation between the microbiota, dietary variable, and clinical marker data sets. The subgenus diversity of Prevotella and Streptococcus was investigated as recently reported (64).
Oligotyping.
Reads assigned to Prevotella and Streptococcus genera were sorted, and entropy analysis and oligotyping were carried out (70). The –C option was set to assess high-entropy nucleotides from Prevotella (19, 95, 97, 99, 106, 110, 177, 179, 220, 223, 263, and 391) and Streptococcus (16, 17, 19, 22, 23, 93, 84, 127, 192, 230, 269, and 299) reads. The result of the analysis led to 83 and 18 oligotype representative sequences for Prevotella and Streptococcus genera, respectively. Subsequently, BLASTn was used for the identification of the representative sequences against the HOMD 16S rRNA RefSeq database, and the top hit was considered for taxonomic assignment.
Data availability.
The 16S rRNA gene sequences produced in this study are available at the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under accession number PRJNA605164.
Supplementary Material
ACKNOWLEDGMENTS
The study was conducted within the diet-induced arrangement of the gut microbiome for the improvement of cardiometabolic health (DINAMIC) project funded within the European Joint Programming Initiative: a Healthy Diet for a Healthy Life (JPI HDHL)–Joint Action Intestinal Microbiomics. The DINAMIC national funding organization is the Italian Ministry of Education, University and Research (prot.0000426).
We thank Agria Spa, Fiammante Icab Spa, Olio Dante Spa, and Pastificio Di Martino Spa for kindly providing foods to be included in the food boxes for our participants. We also thank Ilario Mennella for assistance in the intervention protocol and Maria Assunta Gallo and Centro Diagnostico San Ciro (Portici, Italy) for their support with the clinical visits.
A full list of the DINAMIC consortium and their affiliations appears in the supplemental material.
Funding Statement
The study was conducted within the Diet-Induced Arrangement of the gut Microbiome for the Improvement of Cardiometabolic health (DINAMIC) project funded within the European Joint Programming Initiative "A Healthy Diet for a Healthy Life" (JPI HDHL) - Joint Action "Intestinal Microbiomics".
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Paster BJ, Olsen I, Aas JA, Dewhirst FE. 2006. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 2.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIH HMP Working Group, Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. 2009. The NIH human microbiome project. Genome Res 19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francavilla R, Ercolini D, Piccolo M, Vannini L, Siragusa S, De Filippis F, De Pasquale I, Di Cagno R, Di Toma M, Gozzi G, Serrazanetti DI, De Angelis M, Gobbetti M. 2014. Salivary microbiota and metabolome associated with celiac disease. Appl Environ Microbiol 80:3416–3425. doi: 10.1128/AEM.00362-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piombino P, Genovese A, Esposito S, Moio L, Cutolo PP, Chambery A, Severino V, Moneta E, Smith DP, Owens SM, Gilbert JA, Ercolini D. 2014. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS One 9:e85611. doi: 10.1371/journal.pone.0085611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maukonen J, Mättö J, Suihko M-L, Saarela M. 2008. Intra-individual diversity and similarity of salivary and faecal microbiota. J Med Microbiol 57:1560–1568. doi: 10.1099/jmm.0.47352-0. [DOI] [PubMed] [Google Scholar]
- 7.De Filippis F, Vannini L, La Storia A, Laghi L, Piombino P, Stellato G, Serrazanetti DI, Gozzi G, Turroni S, Ferrocino I, Lazzi C, Di Cagno R, Gobbetti M, Ercolini D. 2014. The same microbiota and a potentially discriminant metabolome in the saliva of omnivore, ovo-lacto-vegetarian and vegan individuals. PLoS One 9:e112373. doi: 10.1371/journal.pone.0112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson L, Holgerson PL, Johansson I. 2017. Saliva and tooth biofilm bacterial microbiota in adolescents in a low caries community. Sci Rep 7:5861. doi: 10.1038/s41598-017-06221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello EK, Carlisle EM, Bik EM, Morowitz MJ, Relman DA. 2013. Microbiome assembly across multiple body sites in low-birthweight infants. mBio 4:e00782-13. doi: 10.1128/mBio.00782-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM, Li H, Alekseyenko AV, Hayes RB, Ahn J. 2016. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 10:2435–2446. doi: 10.1038/ismej.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. 2012. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol 13:R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idris A, Hasnain SZ, Huat LZ, Koh D. 2017. Human diseases, immunity and the oral microbiota—insights gained from metagenomic studies. Oral Sci Int 14:27–32. doi: 10.1016/S1348-8643(16)30024-6. [DOI] [Google Scholar]
- 13.Zhou Y, Gao H, Mihindukulasuriya KA, La Rosa PS, Wylie KM, Vishnivetskaya T, Podar M, Warner B, Tarr PI, Nelson DE, Fortenberry JD, Holland MJ, Burr SE, Shannon WD, Sodergren E, Weinstock GM. 2013. Biogeography of the ecosystems of the healthy human body. Genome Biol 14:R1. doi: 10.1186/gb-2013-14-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron SJS, Huws SA, Hegarty MJ, Smith DPM, Mur L. 2015. The human salivary microbiome exhibits temporal stability in bacterial diversity. FEMS Microbiol Ecol 91:fiv091. doi: 10.1093/femsec/fiv091. [DOI] [PubMed] [Google Scholar]
- 15.Acharya A, Chan Y, Kheur S, Jin LJ, Watt RM, Mattheos N. 2017. Salivary microbiome in non-oral disease: a summary of evidence and commentary. Arch Oral Biol 83:169–173. doi: 10.1016/j.archoralbio.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockburn AF, Dehlin JM, Ngan T, Crout R, Boskovic G, Denvir J, Primerano D, Plassman BL, Wu B, Cuff CF. 2012. High throughput DNA sequencing to detect differences in the subgingival plaque microbiome in elderly subjects with and without dementia. Invest Genet 3:19. doi: 10.1186/2041-2223-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu Q-J, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J. 2015. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 19.Slocum C, Kramer C, Genco CA. 2016. Immune dysregulation mediated by the oral microbiome: potential link to chronic inflammation and atherosclerosis. J Intern Med 280:114–128. doi: 10.1111/joim.12476. [DOI] [PubMed] [Google Scholar]
- 20.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vos WM, Engstrand L, Drago L, Reid G, Schauber J, Hay R, Mendling W, Schaller M, Spiller R, Gahan CG. 2012. Human microbiota in health and disease. SelfCare 3:1–68. [Google Scholar]
- 22.Tang WHW, Kitai T, Hazen SL. 2017. Gut microbiota in cardiovascular health and disease. Circ Res 120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sofi F, Abbate R, Gensini GF, Casini A. 2010. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 24.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'Toole PW, Ercolini D. 2016. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 25.De Filippis F, Vitaglione P, Cuomo R, Berni Canani R, Ercolini D. 2018. Dietary interventions to modulate the gut microbiome—how far away are we from precision medicine. Inflamm Bowel Dis 24:2142–2154. doi: 10.1093/ibd/izy080. [DOI] [PubMed] [Google Scholar]
- 26.Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, Giacco R, Mennella I, Ferracane R, Pons N, Pasolli E, Rivellese AA, Dragsted LO, Vitaglione P, Ehrlich DS, Ercolini D. 19 February 2020. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodukula K, Faller DV, Harpp DN, Kanara I, Pernokas J, Pernokas M, Powers WR, Soukos NS, Steliou K, Moos WH. 2017. Gut microbiota and salivary diagnostics: the mouth is salivating to tell us something. Biores Open Access 6:123–132. doi: 10.1089/biores.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendall CL, Mayr HL, Opie RS, Bes-Rastrollo M, Itsiopoulos C, Thomas CJ. 2018. Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr 58:3070–3084. doi: 10.1080/10408398.2017.1351917. [DOI] [PubMed] [Google Scholar]
- 29.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. 2005. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suvan J, D'Aiuto F, Moles DR, Petrie A, Donos N. 2011. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev 12:e381–e404. doi: 10.1111/j.1467-789X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaffee BW, Weston SJ. 2010. Association between chronic periodontal disease and obesity: a systematic review and meta‐analysis. J Periodontol 81:1708–1724. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodson JM, Groppo D, Halem S, Carpino E. 2009. Is obesity an oral bacterial disease? J Dent Res 88:519–523. doi: 10.1177/0022034509338353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahringer SS, Clemente JC, Corley RP, Hewitt J, Knights D, Walters WA, Knight R, Krauter KS. 2012. Nurture trumps nature in a longitudinal survey of salivary bacterial communities in twins from early adolescence to early adulthood. Genome Res 22:2146–2152. doi: 10.1101/gr.140608.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt SC, Ebersole JL. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 35.Lamont RJ, Koo H, Hajishengallis G. 2018. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kageyama S, Takeshita T, Asakawa M, Shibata Y, Takeuchi K, Yamanaka W, Yamashita Y. 2017. Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PLoS One 12:e0174782. doi: 10.1371/journal.pone.0174782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen TH, Kern T, Bak EG, Kashani A, Allin KH, Nielsen T, Hansen T, Pedersen O. 2018. Impact of a vegan diet on the human salivary microbiota. Sci Rep 8:5847. doi: 10.1038/s41598-018-24207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ercolini D, Francavilla R, Vannini L, De Filippis F, Capriati T, Di Cagno R, Iacono G, De Angelis M, Gobbetti M. 2015. From an imbalance to a new imbalance: Italian-style gluten-free diet alters the salivary microbiota and metabolome of. Sci Rep 5:18571. doi: 10.1038/srep18571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodet C, Chandad F, Grenier D. 2007. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathol Biol (Paris) 55:154–162. doi: 10.1016/j.patbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Eley BM, Cox SW. 2003. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol 2000 31:105–124. doi: 10.1034/j.1600-0757.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 41.Coppo E, Marchese A. 2014. Antibacterial activity of polyphenols. Curr Pharm Biotechnol 15:380–390. doi: 10.2174/138920101504140825121142. [DOI] [PubMed] [Google Scholar]
- 42.Basu A, Masek E, Ebersole JL. 2018. Dietary polyphenols and periodontitis—a mini-review of literature. Molecules 23:1786. doi: 10.3390/molecules23071786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohtani M, Nishimura T. 2020. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp Ther Med 19:1507–1510. doi: 10.3892/etm.2019.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong L, Qi X, Huang S, Chen S, Wu Y, Zhao L. 2015. Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch Oral Biol 60:12–22. doi: 10.1016/j.archoralbio.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Tripathi P, Blaggana V, Upadhyay P, Jindal M, Gupta S, Nishat S. 2019. Antioxidant therapy (lycopene and green tea extract) in periodontal disease: a promising paradigm. J Indian Soc Periodontol 23:25–30. doi: 10.4103/jisp.jisp_277_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharath N, Sowmya NK, Mehta DS. 2015. Determination of antibacterial activity of green coffee bean extract on periodontogenic bacteria like Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans: an in vitro study. Contemp Clin Dent 6:166. doi: 10.4103/0976-237X.156036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veloso DJ, Abrão F, Martins CHG, Bronzato JD, Gomes B, Higino JS, Sampaio FC. 2020. Potential antibacterial and anti-halitosis activity of medicinal plants against oral bacteria. Arch Oral Biol 110:104585. doi: 10.1016/j.archoralbio.2019.104585. [DOI] [PubMed] [Google Scholar]
- 48.Bodet C, Piché M, Chandad F, Grenier D. 2006. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J Antimicrob Chemother 57:685–690. doi: 10.1093/jac/dkl031. [DOI] [PubMed] [Google Scholar]
- 49.Widyarman AS, Suhalim OP, Nandary D, Theodorea CF. 2018. Pomegranate juice inhibits periodontal pathogens biofilm in vitro. Sci Dent J 2:101–108. doi: 10.26912/sdj.v2i3.2572. [DOI] [Google Scholar]
- 50.Kaur G, Kathariya R, Bansal S, Singh A, Shahakar D. 2016. Dietary antioxidants and their indispensable role in periodontal health. J Food Drug Anal 24:239–246. doi: 10.1016/j.jfda.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. 2016. The role of nutrition in periodontal health: an update. Nutrients 8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hujoel PP, Lingström P. 2017. Nutrition, dental caries and periodontal disease: a narrative review. J Clin Periodontol 44:S79–S84. doi: 10.1111/jcpe.12672. [DOI] [PubMed] [Google Scholar]
- 53.Woelber JP, Bremer K, Vach K, König D, Hellwig E, Ratka-Krüger P, Al-Ahmad A, Tennert C. 2016. An oral health optimized diet can reduce gingival and periodontal inflammation in humans-a randomized controlled pilot study. BMC Oral Health 17:28. doi: 10.1186/s12903-016-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skoczek-Rubińska A, Bajerska J, Menclewicz K. 2018. Effects of fruit and vegetables intake in periodontal diseases: a systematic review. Dent Med Probl 55:431–439. doi: 10.17219/dmp/99072. [DOI] [PubMed] [Google Scholar]
- 55.Helmerhorst EJ, Oppenheim FG. 2007. Saliva: a dynamic proteome. J Dent Res 86:680–693. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 56.Patil PB, Patil BR. 2011. Saliva: a diagnostic biomarker of periodontal diseases. J Indian Soc Periodontol 15:310–317. doi: 10.4103/0972-124X.92560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. 2017. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord 38:61–67. doi: 10.1016/j.parkreldis.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 58.Ho M-H, Lamont RJ, Xie H. 2017. Identification of Streptococcus cristatus peptides that repress expression of virulence genes in Porphyromonas gingivalis. Sci Rep 7:1413. doi: 10.1038/s41598-017-01551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Groot PF, Belzer C, Aydin Ö, Levin E, Levels JH, Aalvink S, Boot F, Holleman F, van Raalte DH, Scheithauer TP, Simsek S, Schaap FG, Olde Damink SWM, Roep BO, Hoekstra JB, de Vos WM, Nieuwdorp M. 2017. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One 12:e0188475. doi: 10.1371/journal.pone.0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K. 2014. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao E, Mattos M, Vieira GHA, Chen S, Corrêa JD, Wu Y, Albiero ML, Bittinger K, Graves DT. 2017. Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22:120–128. doi: 10.1016/j.chom.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agnoli C, Krogh V, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Frasca G, Pala V, Berrino F, Chiodini P, Mattiello A, Panico S. 2011. A priori-defined dietary patterns are associated with reduced risk of stroke in a large Italian cohort. J Nutr 141:1552–1558. doi: 10.3945/jn.111.140061. [DOI] [PubMed] [Google Scholar]
- 63.Kong X, Ferracane R, De Luca L, Vitaglione P. 2016. Salivary concentration of N-acylethanolamines upon food mastication and after meal consumption: influence of food dietary fiber. Food Res Int 89:186–193. doi: 10.1016/j.foodres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Berni Canani R, De Filippis F, Nocerino R, Laiola M, Paparo L, Calignano A, De Caro C, Coretti L, Chiariotti L, Gilbert JA, Ercolini D. 2017. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow’s milk containing heat-killed Lactobacillus paracasei CBA L74. Appl Environ Microbiol 83:e01206-17. doi: 10.1128/AEM.01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemoine F, Correia D, Lefort V, Doppelt-Azeroual O, Mareuil F, Cohen-Boulakia S, Gascuel O. 2019. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res 47:W260–W265. doi: 10.1093/nar/gkz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eren AM, Maignien L, Sul WJ, Murphy LG, Grim SL, Morrison HG, Sogin ML. 2013. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Methods Ecol Evol 4:1111–1119. doi: 10.1111/2041-210X.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA gene sequences produced in this study are available at the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under accession number PRJNA605164.