Turkey meat is an important source of animal protein, and the industry around its production contributes significantly to the agricultural economy. The microorganisms present in the gut of turkeys are known to impact bird health and flock performance. However, the respiratory microbiota in turkeys is entirely unexplored. This study has elucidated the microbiota of respiratory tracts of turkeys from two commercial flocks raised in parallel throughout a normal flock cycle. Further, the study suggests that bacteria originating in the gut or in poultry house environments influence respiratory communities; consequently, they induce poor performance, either directly or indirectly. Future attempts to develop microbiome-based interventions for turkey health should delimit the contributions of respiratory microbiota and aim to limit disturbances to those communities.
KEYWORDS: commercial turkey, microbiome, turkey gut microbiota, turkey respiratory microbiota
ABSTRACT
Communities of gut bacteria (microbiota) are known to play roles in resistance to pathogen infection and optimal weight gain in turkey flocks. However, knowledge of turkey respiratory microbiota and its link to gut microbiota is lacking. This study presents a 16S rRNA gene-based census of the turkey respiratory microbiota (nasal cavity and trachea) alongside gut microbiota (cecum and ileum) in two identical commercial Hybrid Converter turkey flocks raised in parallel under typical field commercial conditions. The flocks were housed in adjacent barns during the brood stage and in geographically separated farms during the grow-out stage. Several bacterial taxa, primarily Staphylococcus, that were acquired in the respiratory tract at the beginning of the brood stage persisted throughout the flock cycle. Late-emerging predominant taxa in the respiratory tract included Deinococcus and Corynebacterium. Tracheal and nasal microbiota of turkeys were identifiably distinct from one another and from gut microbiota. Nevertheless, gut and respiratory microbiota changed in parallel over time and appeared to share many taxa. During the brood stage, the two flocks generally acquired similar gut and respiratory microbiota, and their average body weights were comparable. However, there were qualitative and quantitative differences in microbial profiles and body weight gain trajectories after the flocks were transferred to geographically separated grow-out farms. Lower weight gain corresponded to the emergence of Deinococcus and Ornithobacterium in the respiratory tract and Fusobacterium and Parasutterella in gut. This study provides an overview of turkey microbiota under field conditions and suggests several hypotheses concerning the respiratory microbiome.
IMPORTANCE Turkey meat is an important source of animal protein, and the industry around its production contributes significantly to the agricultural economy. The microorganisms present in the gut of turkeys are known to impact bird health and flock performance. However, the respiratory microbiota in turkeys is entirely unexplored. This study has elucidated the microbiota of respiratory tracts of turkeys from two commercial flocks raised in parallel throughout a normal flock cycle. Further, the study suggests that bacteria originating in the gut or in poultry house environments influence respiratory communities; consequently, they induce poor performance, either directly or indirectly. Future attempts to develop microbiome-based interventions for turkey health should delimit the contributions of respiratory microbiota and aim to limit disturbances to those communities.
INTRODUCTION
Bacterial communities (microbiota) associated with poultry have extensive influence on bird development (1, 2), health (3–5), and production performance (6, 7). A wealth of information on microbial density and composition in the gastrointestinal tracts of broiler chickens, layer chickens, and turkeys has been generated in the last decade (3, 4, 8–10). Certain bacterial species in the turkey gut are associated with adverse effects, such as increased susceptibility to pathogens (11–13), inefficient feed conversion (1), and suboptimal market weights (6, 14), while other species are correlated with improved health and enhanced performance metrics, including weight gain (1, 6, 15, 16).
Microbiota composition in the turkey gut is dependent on a number of intrinsic and extrinsic factors. Intrinsic factors, such as bird age and the site within the gut (cecum, duodenum, jejunum, ileum, etc.), are good predictors of the diversity and composition of communities in commercial turkeys (6, 17, 18). Gradual parallel composition changes occur in different gut sites as the bird ages (6, 17, 18), reflecting anatomical differences between sites and age-dependent physiological changes. Possible extrinsic factors are farm location (19) and flock-rearing and health management practices (15, 20, 21), including diet, housing conditions (22), litter reuse (10, 23, 24), and vaccination and antibiotic use (25). These factors do cause microbiota composition changes, but the effects of those changes on production performance metrics are variable and, thus, difficult to predict. In particular, we have shown that the gut microbial compositions of multiple chicken flocks of the same breed and raised adjacently may differentiate from one another with age (26), potentially leading to a performance gap between flocks. Such detailed information is lacking for turkey flocks.
A thorough understanding of the gut and respiratory microbiota is critical to deciphering the potential roles of microbiota in pathogen resistance in addition to production performance. Our previous work in chickens demonstrated that the microbiota of the respiratory system substantially overlaps gastrointestinal microbiota (26, 27). We observed that numerous taxa reside simultaneously in the respiratory and gastrointestinal tracts and shift in parallel over time (26, 27). It is vitally important to establish whether these dynamics are true in turkeys as well, as they may indicate how abrupt changes to flock management disrupt both systems.
Currently, there is no exposition of turkey respiratory microbiota that would permit comparison with gut microbiota. Previous studies of turkey respiratory bacteria have focused on specific pathogens, such as Mycoplasma (28, 29) and Ornithobacterium rhinotracheale (30), and have not considered nonpathogenic microbial communities residing therein. In other poultry, different respiratory sites are known to house distinct bacterial communities that develop with age (26, 27, 31, 32), but the contribution of these communities to poultry health is largely unknown. Of particular interest is the microbiota of the upper respiratory system (the nasal cavity and the trachea), as these sites are among the first points of contact for airborne bacteria, including aerosolized fecal bacteria (33). Understanding how upper respiratory microbiota develops alongside gastrointestinal microbiota is critical to the development of effective interventions against transmission of performance-inhibiting microorganisms and to improve poultry health and productivity.
In the current study, we provide respiratory and gastrointestinal microbiota observed in two identical commercial turkey flocks from a vertically integrated turkey production system. The birds were raised in adjacent barns throughout the brood farm stage but were geographically separated for the grow-out/finisher farm stage. This management setup allowed parallel observation of weight gain and microbiota both before and after separation and transfer to new locations.
RESULTS
Health status and performance of the flocks.
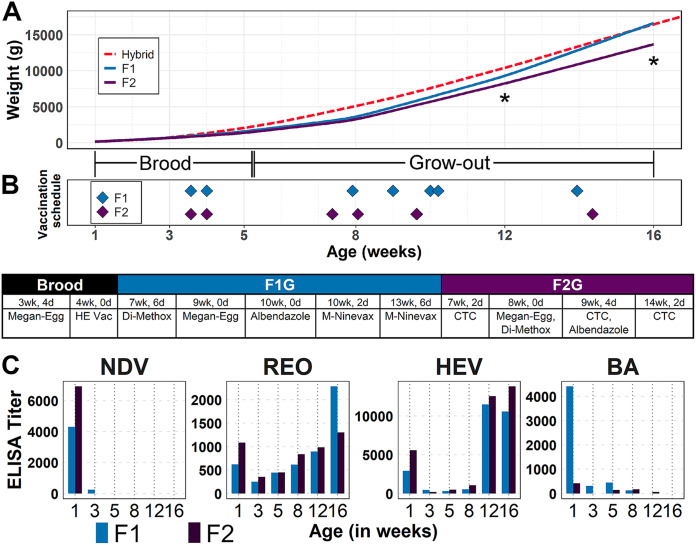
Both flocks were vaccinated against hemorrhagic enteritis virus (HEV) and Salmonella during the brooding stage. During the grow-out/finish stage, while both flocks were booster-vaccinated against Salmonella, flock F1G also was administered Pasteurella multocida vaccine. At 1 week of age, the flocks had high levels of antibodies against HEV, Newcastle disease virus (NDV), reovirus (REO), and Bordetella avium that are presumed to have been passively transferred from the breeder hens to the poults through the egg yolk (Fig. 1B). The maternally derived antibodies subsequently waned to low or undetectable levels unless the flock was vaccinated (HEV) or became infected (REO). Antibody response to HEV vaccination was minimal between 5 and 8 weeks but robust during 12 and 16 weeks. The presence of increasing titers of anti-REO antibodies from the 3rd week onward suggests the flock was infected with reovirus of unknown pathogenicity (Fig. 1B), as has been reported in other commercial flocks (34).
FIG 1.
Flock metadata. Body weights, vaccination timeline, and immune status. Flocks F1 and F2 were raised by the same management during the brood stage (weeks 1 to 5) and separated to two different farms at the grow-out stage (after week 5). (A) Average weights for each flock (F1 in blue, F2 in purple) compared to expected weights for the Hybrid Converter breed (red). Asterisks indicate ages at which bird weights were significantly different between F1 and F2 (P < 0.001). (B) Timing of vaccinations during the sampling period (top) and exact ages and compounds used (bottom). CTC, chlortetracycline. (C) Antibody titers for Newcastle disease virus (NDV), reovirus (REO), hemorrhagic enteritis virus (HEV), and Bordetella avium (BA). Bird ages at sampling are given on the x axes of panels B and C.
We did not determine the immune responses to Salmonella and Pasteurella multocida vaccinations, and no amplicon sequence variants (ASVs) were identified as Salmonella or Pasteurella before or after vaccination. Both flocks were serologically negative for Mycoplasma gallisepticum, M. meleagridis, and M. synoviae, well-known pathogens for turkeys (35). In addition to vaccination, the flocks were treated with albendazole (antihelminthic) and sulfadimethoxine (coccidiostat-antibacterial) as part of a routine management protocol.
Production performance was tracked by taking body weights of the sampled birds. Starting from 5 weeks onward, the average body weights of flock F2 were below the genetic potential of the Hybrid Converter line (36) and remained so until the end of the study timeline (Fig. 1A). A similar trend of lower relative body weight gain was observed in flock F1G until 8 weeks, when the body weights started to gain rapidly and reached genetic potential by 16 weeks. Notably, the average body weight of flock F2G was significantly lighter than that of flock F1G at 12 and 16 weeks (Fig. 1A).
Census of predominant bacterial taxa distinguishes between the gut and respiratory tract environments.
In the cecum, the dominant phyla were Firmicutes and Bacteroidetes, largely represented by the orders Clostridiales and Bacteroidales, respectively (see Fig. S1 in the supplemental material). The most prominent genus was Bacteroides, although Lactobacillus was also common during the brood stage (Fig. S2). In the ileum, the predominant phylum was Firmicutes, with the dominant orders being Clostridiales and Lactobacillales, and the most prominent genus was Lactobacillus. ASVs classified as “Candidatus Arthromitus” were abundant (>5% relative abundance) at 1 week of age (1WOA) but rapidly declined to <1% relative abundance by 12 weeks (Fig. S3A).
Predominant taxa in the respiratory tract of turkeys were classified as Deinococcus, Corynebacterium, and Staphylococcus (Fig. S8 to S11). ASVs from the genera Mycoplasma and Ornithobacterium were abundant in both respiratory sites (Fig. S3B), even though all birds were serologically negative for M. synoviae, M. gallisepticum, and M. meleagridis. At the phylum level, Firmicutes and Actinobacteria predominated in the nasal cavity. The distribution of tracheal phyla through time was not as consistent as that in the other three body sites (Fig. S1); however, there was a much higher prevalence of Proteobacteria than in other sites.
In sum, each body site was characterized by the predominance of a particular genus at all sampling times, Bacteroides in cecum, Lactobacillus in ileum, and Staphylococcus in the nasal cavity, but no genus was consistently predominant in trachea (Fig. S2).
Microbial communities mainly assemble according to body sites and body systems.
The four body sites sampled were measurably different according to several exploratory indices. The total number of ASVs observed was higher in the gut (1,434 in cecum and 1,145 in ileum) than in the respiratory tract (717 in nasal cavity and 689 in trachea) (Fig. S5). However, the average bacterial species richness in each bird was higher in cecum (209 ASVs) and nasal cavity (105 ASVs) and lower in ileum (89 ASVs) and trachea (66 ASVs) (Fig. S5).
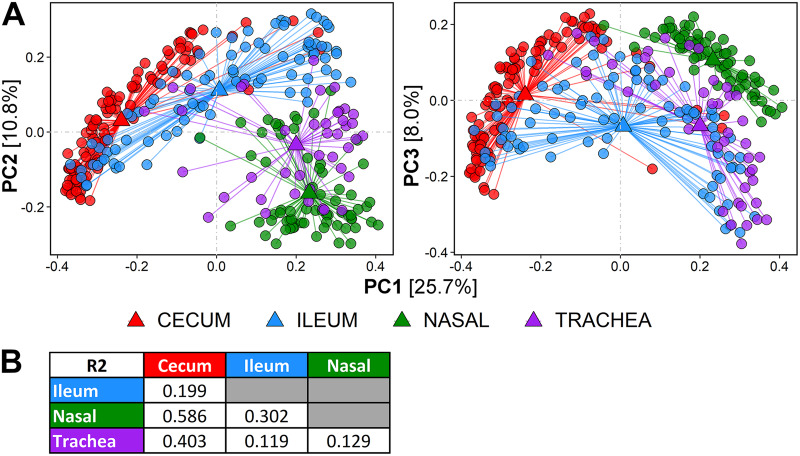
In terms of the overall composition of bacterial communities among the sampled birds, the four body sites are distinct from one another, and the composition of each site changes in various ways as the birds age. Based on principal coordinate analysis (PCoA) of unweighted UniFrac sample distances, samples clustered according to sampling age (P < 0.001 by permutational multivariate analysis of variance [PERMANOVA]) and body site (P < 0.001 by PERMANOVA). Cecum and nasal cavity samples were slightly less dispersed (average distance to centroid of 0.37 and 0.38, respectively, by permutational multivariate dispersion [PERMDISP]) than ileum or trachea samples (average distance to centroid of 0.46 for both by PERMDISP). The distinction between groups is visible in Fig. 2A, with 44.5% of total variance explained (PC1, 25.7%; PC2, 10.8%; PC3, 8.0%). Qualitatively, PC1 separated samples into respiratory tract and cecum, with ileum samples distributed along the range (Fig. 2A), and PC2 stratified samples by age, regardless of body site (Fig. S4A).
FIG 2.
Comparison of bacterial community composition in respiratory and gut sites. (A) Principal coordinate plot of sample distribution according to unweighted UniFrac distances. Axes displayed are PC2 (left plots) and PC3 (right plots), with reference to PC1. Samples are colored according to body site. Values in brackets beside axis labels indicate the percentage of sample variance explained by that axis. Total variance explained by PC1, PC2, and PC3 is 44.5%. (B) R2 values from pairwise PERMANOVA comparison. The R2 value quantifies how well the grouping variable describes the distances between group centroids (triangles). Larger R2 values indicate greater distinction between groups.
Quantitative distinctions between sample groups are specified by PERMANOVA as the amount of variance explained (R2) by the distance between the centroids of pairs of groups. All body sites were significantly distant from one another (P < 0.001 by PERMANOVA for all comparisons) (Fig. 2A), but the R2 values were variable (Fig. 2B). Centroid distances were shorter within the gut (R2 = 0.199) and within the respiratory tract (R2 = 0.129). Cecum and respiratory tract sites were the most distant (R2 with nasal cavity, 0.586; R2 with trachea, 0.403), followed by ileum and nasal cavity (R2 = 0.302). Trachea and ileum were the least distant (R2 = 0.119) (Fig. 2B).
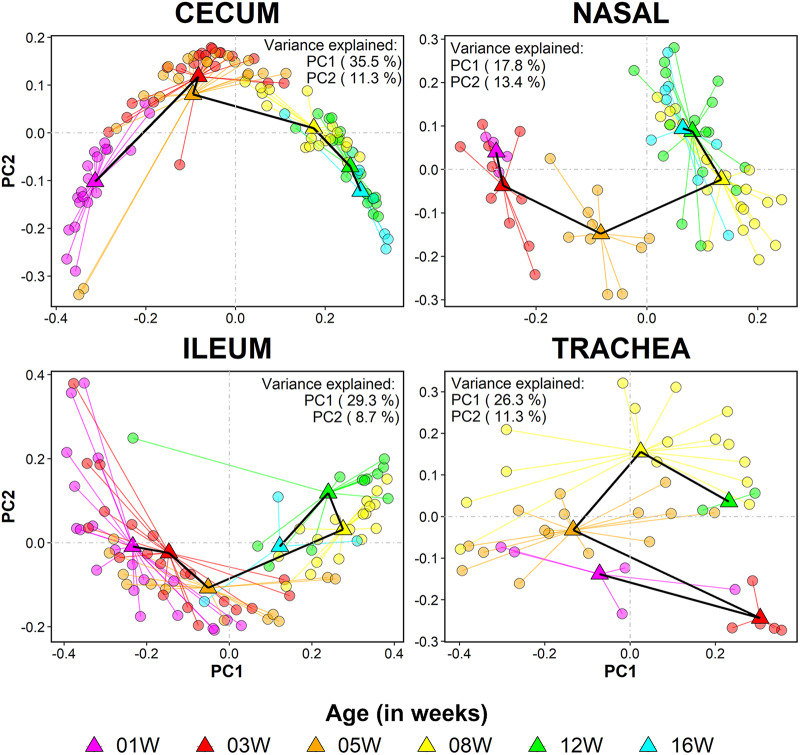
Age had a significant effect on sample grouping when all four body sites were ordinated together (Fig. S4A) and when each body site was ordinated independently (Fig. 3; PERMANOVA, P < 0.001 for all sites). Total variance explained by the first two axes is 44.8% in cecum, 38.0% in ileum, 31.2% in nasal cavity, and 37.6% in trachea. Sample separation between brood (1 to 5 weeks) and grow-out (8 to 16 weeks) stages is visible in all four body sites (Fig. 3).
FIG 3.
Community composition of samples from each body site changes gradually with host age. Principal coordinate plot of sample distribution, given according to unweighted UniFrac distances. Samples (circles) are colored according to age at sampling: 1 week (pink), 3 weeks (red), 5 weeks (orange), 8 weeks (yellow), 12 weeks (green), and 16 weeks (blue). The black line connects the centroids of consecutive age groups. Only the first two axes are given for each plot. Variance explained by each axis is given in the corner of each plot.
Gut and respiratory tract communities do not exist in isolation from each other.
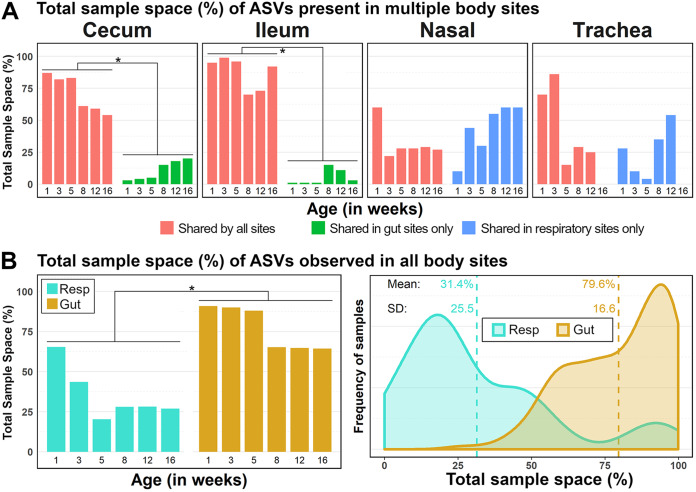
Despite obvious differences in bacterial community composition and species richness between body sites (Fig. 2 and described above), several taxa were shared among them. Of the 1,874 ASVs observed in this study, 285 were shared across all four body sites, 506 were shared only between gut sites (cecum and ileum), and 133 were only shared between respiratory sites (trachea and nasal cavity) (Fig. S5).
The relative abundances of shared and unique ASVs vary according to body site and are summarized in Fig. 4A and in greater detail in Fig. S5. Here, we describe site occupancy in terms of sample space (the maximum of which is 100%), referring to the total relative abundance of each ASV averaged across all samples of the same age group, body site, and flock (unless flock is not indicated in the figure, e.g., Fig. 4). This terminology does not differentiate transient species from long-term inhabitants.
FIG 4.
Total sample space (per age group) occupied by ASVs that are shared by all sites (red; 285/1,874 ASVs), shared by sites in the respiratory tract only (blue; 133/1,874 ASVs), and shared by sites in the gut only (green; 506/1,874 ASVs). Percentages given are the mean total relative abundance of shared ASVs in a body site at a particular sampling age. Paired t tests were performed between the mean total abundance of ASV groups per age in a pairwise fashion with Bonferroni correction for multiple comparisons between ASV groups in a body site (A) and between the respiratory tract and gut (B, left). Asterisks indicate significant differences between ASV groups (P < 0.05). (B, right) The distribution of sample values for total sample space occupied by ubiquitous ASVs is also shown to demonstrate the distinction in respiratory and gut occupancy by these ASVs at the sample level. A Venn diagram of ASV distribution among samples is given in Fig. S5.
Irrespective of age, ASVs that were shared among all body sites (ubiquitous) occupied more sample space in the gut than the respiratory tract (P < 0.01 by t test) (Fig. 4B). The changes in abundance of these taxa over time indicate increasing compositional diversification between the gut and respiratory tract with age. In general, as the amount of sample space occupied by the ubiquitous ASVs decreased with age, there was a complementary increase in the sample space occupied by ASVs unique to gut sites or respiratory sites only (Fig. 4A and Fig. S5).
Flock barn and grow-out stage separation had minimal influence on alpha diversity, except in the nasal cavity.
The flocks were housed in two proximally located barns within the same farm and geographical location during the brood stage, designated F1B and F2B. The birds of these brood barns were destined for different (geographically distant) grow-out farms, designated F1G and F2G, respectively. Age-independent examination of species richness (measured as the number of ASVs observed) and Pielou’s evenness did not significantly differentiate the two flocks in any body site. However, species richness distribution indicated significant differences between the brood and grow-out stages (P < 0.05 by t test) but not between flocks within each stage (P > 0.1 by t test). This pattern was consistent in all four body sites. Linear regression of species richness and evenness against week of age at sampling indicated mostly minor differences between flock groups (Table 1 and Fig. S6). Species richness in individual birds tended to increase with age (b > 0, where b is the slope). Although richness correlated well with age at sampling for gut samples (cecum, R2 > 0.6; ileum, R2 > 0.2), it did not correlate consistently well with respiratory samples. Specifically, species richness in the nasal cavities of F2 birds correlated positively with age at sampling (b = 6.23, R2 = 0.53) but fluctuated frequently in F1 birds (b = −0.24, R2 < 0.01). The slopes of each correlation were not statistically different between F1 and F2 in cecum, ileum, or trachea but were significantly different in nasal cavity (P < 0.001 by t test) (Table 1 and Fig. S6). Bacterial community evenness in individual birds tended to increase with increasing sampling age, but the correlations generally were not as strong as those with richness. The changes in evenness over time in the ileum and trachea were not significantly different between F1 and F2 birds. However, significant differences were seen in the cecum and nasal cavity (Table 1 and Fig. S6).
TABLE 1.
Coefficients for linear regression models between richness and evenness indices and bird sampling agea
| Body site and index | Flock | b | A | R2 | df | v | t | P value |
|---|---|---|---|---|---|---|---|---|
| Richness | ||||||||
| Cecum | F1 | 20.7 | 79.6 | 0.863 | 51 | 1.33 | 1.07 | 0.224 |
| F2 | 16.4 | 103 | 0.663 | 51 | 2.68 | |||
| Ileum | F1 | 13.6 | 20.2 | 0.549 | 39 | 3.90 | 0.70 | 0.310 |
| F2 | 8.32 | 37.2 | 0.26 | 44 | 4.48 | |||
| Nasal cavity | F1 | −0.24 | 105 | 0.00159 | 33 | 1.10 | 9.97 | 1.73E−14 |
| F2 | 6.23 | 56 | 0.53 | 33 | 1.04 | |||
| Trachea | F1 | 4.3 | 59 | 0.0561 | 21 | 14.8 | 1.09 | 0.217 |
| F2 | 3.77 | 25.6 | 0.0851 | 19 | 8.04 | |||
| Evenness | ||||||||
| Cecum | F1 | 0.00475 | 0.698 | 0.196 | 51 | 1.81E−06 | 3.12 | 0.004 |
| F2 | 0.00916 | 0.647 | 0.227 | 51 | 5.60E−06 | |||
| Ileum | F1 | 0.0216 | 0.482 | 0.337 | 39 | 2.35E−05 | 0.34 | 0.375 |
| F2 | 0.00966 | 0.5 | 0.077 | 44 | 2.54E−05 | |||
| Nasal cavity | F1 | −0.00268 | 0.645 | 0.0351 | 33 | 5.98E−06 | 9.49 | 1.20E−13 |
| F2 | 0.0101 | 0.573 | 0.5 | 33 | 3.09E−06 | |||
| Trachea | F1 | 0.0116 | 0.425 | 0.0163 | 21 | 3.87E−04 | 0.65 | 0.319 |
| F2 | 0.00629 | 0.371 | 0.00723 | 19 | 2.86E−04 |
Coefficients given are slope (b), y intercept (A), and coefficient of determination (R2). Also given are the degrees of freedom for the group (df = n − 1), variance around the slope (v; see function 1), and an associated value for Student’s t test comparing the two slopes (t; see function 2), from which a P value is derived.
Bacterial community compositions were differentiated after the flocks transitioned to the grow-out barns.
Like species richness and evenness, the differences in bacterial species composition between birds (as measured by unweighted UniFrac distances) visibly changed with age in all body sites (Fig. 3). However, bacterial communities of each body site in the two flocks were compositionally more similar at the brood stage than at the grow-out stage in the cecum, ileum, and nasal cavity (P < 0.05 by PERMANOVA) (Table 2 and Fig. S7). In contrast, tracheal composition was more differentiated at the brood stage than in the grow-out stage. The composition of each body site was further examined by focusing on the dynamics of several predominant ASVs across age groups. ASVs were considered predominant if they occurred in 50% of birds within an age group, were retained from emergence until 16 weeks, and achieved a relative abundance of >3.5% in at least one age group. The relative abundances of constituent bacteria that were predominant in both groups during the brood stage were differentially decreased by the acquisition of new ASVs at the start of the grow-out stage (i.e., posttransfer to new farms) (Fig. S8 to S11).
TABLE 2.
Statistical comparisona of unweighted UniFrac distances between flocks and farm stages
| Body site |
R2 valuec |
|||
|---|---|---|---|---|
| Between brood (F1B vs F2B) | Between grow-out (F1G vs F2G) | Brood (F1B + F2B) versus grow-out (F1G + F2G) | Between flock (brood + grow-out) | |
| Cecum | 0.006 | 0.276b | 0.412b | 0.049 |
| Ileum | 0.026 | 0.148b | 0.403b | 0.023 |
| Nasal cavity | 0.020 | 0.134b | 0.327b | 0.013 |
| Trachea | 0.195b | 0.157 | 0.084b | 0.015 |
PERMANOVA coefficient of determination (R2) describing variance explained by the segregation of groups in composition space (see also Fig. S7).
Significant difference from PERMANOVA (1,000 iterations, P < 0.05).
Brood, 0 to 5 weeks; grow-out, >5 weeks; flock, F1B + F1G and F2B + F2G.
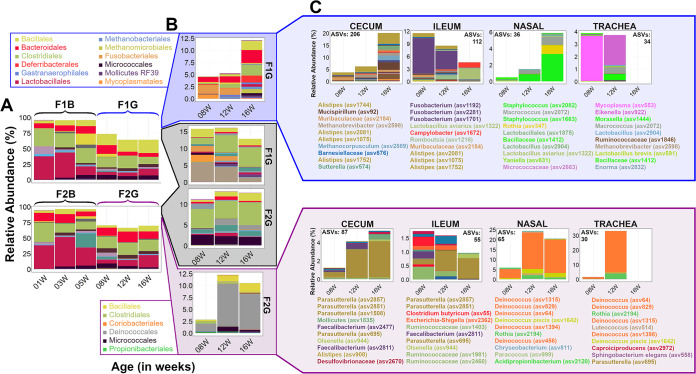
Generally speaking, a greater number of bacteria gained predominance at the grow-out stage in the respiratory tract (particularly in trachea) than in the gut, regardless of flock group. Most predominant genera in the nasal cavity persisted from 1WOA (F1 and F2, Brevibacterium, Corynebacterium, Lactobacillus, Rothia, and Staphylococcus; F1, Kocuria; F2, Macrococcus). From 8 weeks, F2 acquired Deinococcus.
In general, far fewer predominant taxa persisted long-term in trachea than in the nasal cavity. Members of the Burkholderiaceae, Escherichia, Lactobacillus, Staphylococcus (F1 and F2), Bifidobacterium, and Streptococcus (F1) all appeared in the trachea prior to farm transfer. Corynebacterium (F1 and F2), Macrococcus (F1), Brevibacterium, Deinococcus, and Rothia (F2) emerged and persisted in trachea from 8 weeks (after the transfer) (Fig. S8 to S11). Strikingly, Deinococcus became predominant only in the respiratory tracts of F2G birds (Fig. S10 and S11). Differences in Deinococcus abundance between groups were tested with Dunn’s Kruskal-Wallis rank sum test and Holm’s correction for multiple comparisons. The abundance of Deinococcus in nasal and tracheal samples was not different between F1B, F2B, and F1G (P > 0.2). Conversely, Deinococcus abundance was significantly higher in F2G than in the other groups in both nasal and tracheal samples (P < 0.009).
In the gut, genera that tended to emerge at 1WOA and persist through 16WOA were Bacteroides, Lactobacillus, Ruminococcus, Bifidobacterium, and Escherichia. Genera that emerged as predominant in the gut after farm transfer (8 to 16WOA) were Phascolarctobacterium, Sutterella, Alistipes, and Romboutsia (Fig. S8 to S11). Although several genera predominated simultaneously between flocks, the individual ASVs representing those genera in the gut and respiratory tract were rarely shared between flocks.
New acquisition of unique taxa coincides with the transfer to new farms.
Differences between groups were minimal during the brood stage but increased after transfer to grow-out barns: ASVs shared between the four flock groups (483/1,874) occupied nearly 100% of available sample space during the brood stage, but that percentage was reduced in both flocks after the move (Fig. 5A). New ASVs were acquired in both flocks after the farm transfer, 489 of which were common to both flocks, 273 of which were unique to F1G, and 161 of which were unique to F2G (Fig. 5B; see Fig. S12 for the most abundant ASVs in each flock and Data Sheet S1 for a complete ASV list). The most abundant ASVs (at >1% mean relative abundance) unique to F1G were classified as Fusobacterium, Alistipes, Mycoplasma, Mucispirillum, Staphylococcus, Lactobacillus avarius, Eikenella, Muribaculaceae, Methanobrevibacter, Macrococcus, Campylobacter, Methanocorpusculum, Rothia, and Romboutsia (Fig. 5C). The most abundant ASVs (at >1% mean relative abundance) unique to F2G were classified as Deinococcus, Deinococcus piscis, Rothia, and Parasutterella (Fig. 5C).
FIG 5.
Dynamics of unique bacterial taxa that are acquired by each flock independently during the grow-out period. (A) The total sample space occupied by ASVs that were common to both flocks during both the brood and grow-out stages. Colors represent different taxonomic orders. Note that the sample space occupied is close to 100% in the brood stage but is depressed in the grow-out stage. (B) The majority of the remaining sample space is occupied by ASVs either shared among the grow-out flocks (black box) or unique to each (blue and purple boxes). Colors represent different taxonomic orders, the most abundant of which are given in the adjacent legends. (C) The total relative abundance of ASVs unique to each flock, distributed between the 4 body sites. Legends below each bar chart name the top 10 most abundant of these ASVs within each body site. Colors represent the lowest taxonomic level assigned to an ASV by the SILVA classifier.
Potentially pathogenic bacteria identified in both flocks.
We identified two ASVs that closely matched M. gallisepticum, M. tullyi, and M. imitans when referenced against the NCBI BLAST 16S rRNA gene database (Fig. S3 and S12). These two ASVs likely represent M. tullyi (37) or M. imitans (38, 39), since both flocks were serologically negative for M. gallisepticum. Both Mycoplasma ASVs were present only at 8 weeks. Additionally, the common respiratory pathogen Ornithobacterium rhinotracheale was observed as a single ASV that matched the BLAST reference for O. rhinotracheale with 100% identity in both flocks. O. rhinotracheale occupied 68.9% of tracheal sample space in F2 at 5 weeks, dropping to 22.1% at 8 weeks, while occupying 7.2% of sample space in F1 (8 weeks) (Fig. S3). Although both flocks were characterized with below-average weights throughout the brood and grow-out periods (Fig. 1A), the incidence of O. rhinotracheale and Mycoplasma in trachea corresponded to further weight gain suppression during weeks 5 and 8 (Fig. S3C).
DISCUSSION
Information on the turkey respiratory tract microbiota is currently limited, as no culture-independent surveys have been conducted previously to the best of our knowledge. This study provides an extensive census of bacterial residents in the respiratory tract (trachea and nasal cavity) of turkeys, in conjunction with the gut (ileum and cecum), in the course of a typical commercial flock cycle. Bacterial community compositions among the body sites formed a gradient, with highly structured (more complex) communities (cecum and nasal cavity) at the extremes and less structured (less complex) communities (ileum and trachea) in between (Fig. 2). This gradient closely recapitulates our recent observations in layer and broiler chickens (26, 27). Additionally, there was substantial sharing of bacterial taxa between the gut and respiratory tract (Fig. 4; see also Fig. S5 in the supplemental material). The sample space occupied by these taxa was significantly larger in the gut than in the respiratory tract (Fig. 4), suggesting that several of these taxa colonize the gut and migrate to the respiratory tract as aerosolized fecal matter. What is yet unclear is how many of those migrating taxa are ephemeral occupants versus long-term inhabitants of respiratory mucosal layers. The baseline data generated in this study will guide future controlled experiments designed to uncover causal relationships between respiratory microbiota, gut microbiota, and turkey flock performance.
The presence or absence of certain potential respiratory pathogens coincided with the emergence of below-average weight during the late brood and early grow-out period (5 to 8 weeks). For instance, the respiratory pathogen O. rhinotracheale (1 ASV) (35, 40, 41) occupied >60% of tracheal sample space in F2 at 5 weeks (Fig. S3B), a trend that diminished with subsequent samplings but likely influenced the stall in weight gain recovery in that flock (42). Mycoplasma could be significant in the context of below-average rates of weight gain observed in flocks (29). Although serological evidence precluded the presence of antibodies to M. gallisepticum, M. meleagridis, and M. synoviae, flock F2 began receiving precautionary treatment with chlortetracycline at 7 weeks. This treatment may account for the low relative abundance of Mycoplasma ASVs in F2 relative to F1 at 8 weeks (Fig. S3B), yet the abundance of Mycoplasma in each flock inversely corresponded to disparities in average weight (Fig. 1). The effects of chlortetracycline on composition may be compounded by the effects of the farm move, resulting in a prolonged period of microbiota disruption.
After 8 weeks, the flocks began to differentiate in their trajectories of weight gain (Fig. 1A) and, at the same time, gained several bacterial taxa unique to each flock (Fig. 5). In F1G, the trend of weight recovery visible from 12 weeks coincided with Staphylococcus increase in the nasal cavity. The novel Staphylococcus ASVs (Fig. 5) in the nasal cavity of F1G joined a large extant population of these bacteria (Fig. S10). Although several Staphylococcus species are known pathogens (43), the abundance of Staphylococcus increased as bird weight improved in F1 and remained suppressed in F2 (where bird weight did not improve). This suggests that some Staphylococcus species behave commensally, potentially by preventing the colonization of the mucosal layer by naive environmental bacteria.
The lack of weight recovery in F2G also coincided with the emergence and persistence of numerous Deinococcus ASVs in the respiratory tract (Fig. 1A and 5). Deinococcus has been previously identified as a common extraction kit reagent contaminant, particularly in low-biomass samples (44). Given that samples from the respiratory tract typically are low biomass (compared to gut content samples) (44), the prevalence of this genus in the respiratory samples of F2G initially was cause for concern. However, birds of the same age group from both flocks were necropsied at the same time, and the processed samples were extracted utilizing the same aliquots of reagents. Under these conditions, contaminants are likely to be well distributed among samples processed together, and this was not observed. The significantly greater abundance of Deinococcus in F2G suggests a contaminant in the housing itself that may be inhabiting mucosal surfaces in the upper respiratory tract. Deinococcus species have been previously isolated in a number of poultry environments, including rearing houses and carcass processing facilities, even after decontamination (45, 46). This genus also was shown to carry the tetracycline efflux transporter gene required for resistance to chlortetracycline (47), which suggests that it expanded as chlortetracycline-susceptible taxa declined in this flock (Fig. 1). Its effects on poultry health have not been explored in depth, although one study reported that supplementing layer hen feed with dried-fermented powder of Deinococcus improved feed conversion and egg yolk color (102). Their pervasiveness in the respiratory system ultimately may be innocuous, but any causal relationship with weight suppression and possible mechanisms of disruption can only be elucidated under controlled conditions.
The associations of gut microbiota with poultry performance metrics are well-studied (6, 48–50). Changes in the abundance of certain taxa after a disturbance (in this case, transfer to new barns or farms) are often associated with performance issues and pathogen infections (3, 5, 22, 25, 51). For example, the rapid decline of “Candidatus Arthromitus” in ilea of both flocks during the brood stage (Fig. S3) corresponded qualitatively with the emergence and persistence of below-average weights (Fig. 1). A causal relationship between “Candidatus” extinction and poor weight gain in young poults is likely, since this taxon was previously found to be dominant in turkey flocks characterized as heavy (6, 16, 25).
As in the trachea and nasal cavity, several gut bacteria were uniquely acquired by each flock at the grow-out farms. Fusobacterium ASVs were abundant in flock F1 ilea at 8 weeks (when weight gain was most suppressed) and gradually diminished to very low levels by 16 weeks (when average bird body weight reached the expected weight) (Fig. 1 and 5). F2G experienced noticeable increases of Parasutterella ASVs in cecum and ileum, as body weights failed to recover between 8 and 16 weeks. Both Fusobacterium and Parasutterella spp. have been identified in the poultry gut (48, 52–54) but with no overt links made to poor performance. However, both genera have been associated with pathogenic effects in humans and other species (55–58). It might be worthwhile to examine these genera more closely with reference to potential correlations to poultry weight or other metrics of performance.
Beyond the potential associations with trajectories of weight gain, important insights into turkey microbiota beta diversity were indicated. First, this study corroborates previous observations that turkey age is a discernible driver of compositional change in gut sites (Fig. 3) (6, 17, 18). Age is also an important driver of respiratory microbiota but more so in the nasal cavity than in trachea. Second, although communities of all body sites shifted significantly with the farm move (Table 2 and Fig. S7), differentiation between the flocks was observed only in the gut and nasal cavity. This indicates that the avian gut and nasal cavity environments are more hospitable to long-term inhabitants than the trachea (59). Community composition in the avian trachea may be mostly ephemeral and turn over species more frequently, as observed in the human respiratory tract (60–62).
In contrast with beta diversity, alpha diversity trends with age were notably different between the gut and respiratory sites. In cecum and ileum, species richness increased as linear functions of age that did not significantly differ between flocks (Table 1). Bacterial alpha diversity change with age in gut sites was shown previously in poultry and humans (63–65), but evidence of similar trends in poultry respiratory sites is lacking. In this study, neither the nasal cavity nor the trachea demonstrated a consistent linear relationship between alpha diversity (species richness and evenness) and turkey age (Table 1). After the farm transfer, diversity in the nasal cavity remained constant in F1 but suddenly increased in F2, possibly as a result of high diversity in the barn environment serving as the source population (23, 24, 66). Tracheal diversity was not as systematically affected by the farm transfer in either flock, although several nasal bacteria were also observed in the trachea. At this time, it is unclear whether one of the two sites acquired bacteria first or whether they acquired bacteria simultaneously and share continuously. Further study of microbial exchanges between respiratory sites is fundamental for the development of intervention strategies toward reducing pathogen load and recuperating poor performance.
It should be noted that while age-dependent microbial dynamics in different body sites are the conventional focus of poultry microbiome research and are significant drivers of compositional variation (6, 18, 21, 26, 27, 31, 32, 53, 67), the factors “body site” and “age” in these data accounted for just 25.6% of the total compositional variation between individuals within the flock. Multivariate regression model fitting with PERMANOVA indicated that individual birds accounted for 26.2% of sample variance, while age, body site, and flock designation accounted for 20.3%, 5.3%, and 1.9% of sample variance, respectively. Studies in other avian species and in humans have also indicated that intrinsic factors, such as species, age, sex, or body site, can be significant according to PERMANOVA while accounting for little overall variance (68–72). In this instance, the large proportion of residual variance (46.4%) indicates influences from additional factors, such as variations in management, heterogeneity in the metacommunity around the birds in a field setting (73), and the introduction of artificial variation from the sampling methods used (44, 74, 75) or DNA extraction kits and protocols. For instance, although the kits used in this study were previously optimized for gut and respiratory samples (27), using different kits for different sample types can artificially introduce sample variance.
Nevertheless, this study has provided necessary data to set the groundwork for further studies that will enhance our understanding of the turkey respiratory microbiome. Extensive metadata were collected throughout the study to track the flocks with regard to vaccination history, immune status, antibiotic treatment, and weight gain trajectories. Despite our attempt to establish correlations or associations between microbiota and different metadata parameters, the uncontrolled field study has several limitations. Overlapping vaccination schedules impeded direct analysis of the effects of the vaccines on the microbiome, such as induction of inflammatory responses in the mucosal environment that may disrupt local microbial communities (76, 77). Likewise, we could not determine how microbiota responds to reovirus infection or other pathogens, because the precise time of the natural infection was unknown, especially since the birds were also given live vaccines. Furthermore, the scope of this study did not accommodate the assessment of different turkey breeds and production systems, such as organic and pasture methods. In addition, although the QIIME 2 bioinformatics pipeline has allowed the clustering of 16S rRNA gene reads into biologically meaningful ASVs, the curation of the reference databases (such as SILVA) used for taxonomic classification still is not rigorous enough to guarantee unequivocal taxonomic assignments. Likewise, the 16S rRNA gene sequencing and the sampling and sample processing methods employed in this study did not differentiate transient or dead occupants from live and active colonizers of the sampled sites. 16S rRNA barcoding methods, in conjunction with microbial metabolomics profiles, are one means of better phylotyping the resident microbiome (78).
Conclusions.
This study has presented a detailed picture of turkey respiratory microbiota over the course of a normal commercial turkey farm sequence. Body site and bird age together account for very little compositional variance between samples, suggesting that extrinsic factors (environmental and managerial) influence beta diversity more substantially. Nevertheless, comparison of the composition and temporal dynamics of tracheal, nasal, and gut microbiota in separate flocks has suggested several testable hypotheses: (i) that gut microbiota highly influence the occupation and pathogen susceptibility of respiratory mucosae via the surrounding environment; (ii) that community structure in the nasal cavity is more stable than that in the trachea and, therefore, is more easily manipulated; (iii) that community assembly in the nasal cavity continuously influences what the trachea is exposed to; and (iv) that changes in immune status (via infection or vaccination) disrupt both the gut and respiratory microbiota, regardless of the site of infection. Additionally, by taking advantage of natural differences in the rates of flock weight gain trajectories, associations with poor flock performance were attributable to just a few taxa in both respiratory and gut sites. Future studies should focus on defining the effects of these taxa and elucidating the mechanisms of interfacing that may exist between the sites themselves, including influences from the environment.
MATERIALS AND METHODS
Flock management and sampling.
Two flocks of commercial Hybrid Converter turkeys, each having more than 11,000 birds, were sampled for an entire production cycle between November 2015 and March 2016. The birds raised on this production cycle were all male. The birds were raised on a floor with wood shavings as litter and provided ad libitum access to feed and water. Feed formulations for each of the brood and grow-out periods were not provided by the company, as they are proprietary information, but were consistent between the two flocks. The flocks were reared in two adjacent barns (generically designated F1 and F2) during the brood period, i.e., from hatch to 5 weeks of age. After this period, each flock was moved to a different grow-out farm (Fig. 1). All farms are subsidiary to the same turkey production company and, therefore, are subject to the same management protocols and feeding regimens (including feed formulations). When referring to the flocks specifically during the brood stage, they are designated F1B and F2B. Likewise, when referring to the flocks during the grow-out stage, they are designated F1G and F2G, respectively.
Vaccinations during the brood period were performed on the same days for both flocks. The birds were vaccinated with AviPro Megan Egg (Elanco Animal Health Inc., Greenfield, IN, USA; against Salmonella enterica serovar Typhimurium) at 3 weeks and 4 days of age and with H.E. Vac (Arko Labs, Ltd., Jewell, IA, USA; against hemorrhagic enteritis virus) at 4 weeks. During the grow-out period, the two farms administered vaccines and antimicrobials independently based on routine management protocol. Flock F1G was treated with AviPro Megan Egg at 9 weeks, albendazole (Zoetis Inc., Parsippany, NJ, USA; for deworming) at 10 weeks, and two doses of M-NINEVAX-C (Intervet Inc., Millsboro, DE, USA; against Salmonella and Pasteurella multocida) at 10 weeks and 2 days of age and again at 13 weeks and 6 days of age. Flock F2G was treated with chlortetracycline (Zoetis Inc., Parsippany, NJ, USA) at 7 weeks and 2 days, at 9 weeks and 4 days, and at 14 weeks and 2 days as a prophylactic measure against Mycoplasma infection. F2G was also given AviPro Megan Egg at 8 weeks and albendazole at 9 weeks and 4 days. Both grow-out flocks were administered anticoccidial sulfadimethoxine (Di-Methox; Huvepharma, Inc., Peachtree City, GA, USA) in drinking water (Fig. 1).
A total of 104 birds were sampled at 3 time points during brooding (1, 3, and 5 weeks) and grow-out (8, 12, and 16 weeks). The sample sizes were the following: 10 birds per flock at each time point during brooding, 10 birds per flock at 8 weeks, 8 birds per flock at 12 weeks, and 4 birds per flock at 16 weeks (see Table S1 in the supplemental material). Blood was drawn from live birds via the brachial (wing) vein for serum collection prior to euthanasia. The birds were humanely euthanized in accordance with protocol number 2015A00000056-R1, approved by The Ohio State University Institutional Animal Care and Use Committee. In brief, the animals were exposed to carbon dioxide (CO2) in a euthanasia chamber (1 to 3 birds, depending on bird size). The CO2 flow was maintained at 10 to 30% displacement of chamber volume/minute until euthanasia was complete. Euthanasia was confirmed by the absence of breathing and lack of heartbeat. After euthanasia, the body weight of each bird was measured separately prior to tissue excision.
Detection of vaccine- and pathogen-specific serum antibodies.
Serum samples were sent to the Animal Disease Diagnostic Laboratory, Ohio Department of Agriculture (Columbus, Ohio), for antibody quantification. Levels of reovirus antibodies in serum were measured by enzyme-linked immunosorbent assay using an IDEXX REO Ab test kit (IDEXX, Westbrook, ME, USA). Antibodies against the Newcastle disease virus (NDV), hemorrhagic enteritis virus (HEV), Mycoplasma meleagridis (MM), Mycoplasma synoviae (MS), and Mycoplasma gallisepticum (MG) were quantified or detected with ProFLOK NDV T Ab, HEV Ab, MM-T Ab, and MG-MS Ab kits (Zoetis), respectively.
Sample collection, processing, DNA extraction, and 16S rRNA gene sequencing.
The ileum and cecum each were excised from the birds using sterile scissors and forceps. The tissues were placed in sterile Falcon tubes and immediately snap-frozen with liquid nitrogen. Deep-frozen cecum and ileum tissues were homogenized using mortar and pestle and subsampled to 0.3 g total organic mass. DNA extraction for ileum and cecum homogenate (0.3 g) was performed using the DNeasy PowerSoil kit (Qiagen Sciences Inc., Germantown, MD).
The trachea was excised inside the biosafety cabinet and flushed by repeatedly drawing and expelling phosphate-buffered saline (PBS) through the length of the trachea (from both directions) with a filtered pipet tip. The nasal cavity was washed during necropsy by flushing and retrieving 150 μl PBS through the nares with a 200-μl filtered pipet tip. This process was repeated until about 1 ml of the total volume of PBS containing organic material was collected. Both tracheal and nasal wash collection solutions were centrifuged until a pellet of organic material formed at the bottom of the tube (10,000 × g for 5 min). DNA extraction for tracheal and nasal wash pellets was performed using the DNeasy blood and tissue kit (Qiagen Sciences, Inc., Germantown, MD).
Samples of the same age and body site were extracted together, regardless of the flock designation. DNA was quantified using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and standardized to concentrations of up to 100 ng/μl in a total volume of 20 μl/sample. The 16S rRNA gene barcoding of the V4 region (covered by the 515F [GTGYCAGCMGCCGCGGTAA] and 806R [GGACTACNVGGGTWTCTAAT] primer pair) was performed with Illumina MiSeq (reagent kit v3; Illumina, San Diego, CA) by the University of Minnesota Genomics Center (Minneapolis, MN). Cecum, ileum, and trachea wash samples were not pooled prior to sequencing. To increase biomass for sequencing, nasal wash samples from 1 week were pooled into four pools (5 birds/pool, 2 pools/flock), and nasal wash samples from 3 weeks were pooled into ten pools (2 birds/pool, 5 pools/flock) (Table S1). Further details on collection, modifications to the default extraction protocols, PCR, and sequencing conditions were previously reported by Ngunjiri et al. (27).
Sequence processing and feature table generation and filtering.
The barcoded Illumina sequencing data were demultiplexed to generate fastq files for each sample. Proximal and distal primers and adapters were trimmed from the reads using BBDuk, and paired-end reads were merged using BBMerge, both from the BBTools (v35) suite of DNA/RNA analysis software provided freely by the Joint Genome Institute (79). 16S rRNA amplicon primers were removed using Cutadapt (v1.4.2) (79, 80). Sequences of lengths less than 245 and greater than 260 bp were filtered using the BBMap module of BBTools.
The sequences were imported into QIIME2 (2019 v1.0) (81) in Casava 1.8 single-end demultiplexed format for further processing using different algorithms (implemented in QIIME2). Imported sequences were denoised with DADA2 (74) using the pooled-sample chimera filtering method. We used VSEARCH for the detection of chimeric sequences and open-reference clustering of amplicon sequence variants (ASVs) with 100% identities using the SILVA (release 132) reference database (82). The feature table produced from this step contained ASV counts for each sample. VSEARCH (83) was also used to identify non-16S rRNA gene ASVs, which were subsequently removed from the feature table. The table was filtered further to remove ASVs with a total frequency of <10 (in the entire table) and those present in only 1 sample. The representative set of sequences next was aligned de novo using MAFFT. Nonconserved and highly gapped columns of the alignment were removed by applying the “mast” option of the alignment plugin. The aligned filtered sequences then were used to reconstruct a phylogenetic tree using FastTree, which was rooted at the midpoint prior to application in beta diversity analysis.
For taxonomic classification, we trained a naive Bayes classifier (84) on reference sequences extracted from the SILVA 16S rRNA gene database (release 132) based on matches to the 515F and 806R primer pair (85) and a taxonomic identification table (majority classification) based on reference sequences clustered at 99% identity. Note that the designation of taxon names in the SILVA classifier is based on complete 16S rRNA sequences (i.e., the entire 16 rRNA gene) and, therefore, is compliant with the guidelines published by Yarza et al. (86). Lastly, the classifier was used to assign taxonomy to the ASVs observed in our experimental data.
The final feature table was rarefied to 5,000 reads per sample. Samples with read counts under this amount were discarded (Table S1). All further analyses were conducted in RStudio (87) with the following packages: BiocManager (88), biomformat (89), dplyr (90), reshape2 (91), ape (92), ggplot2 (93), gridExtra (94), RColorBrewer (95), ggpubr (96), xlsx (97), GUniFrac (98), vegan (99), VennDiagram (100), and pairwiseAdonis (101). Samples of the same body site, age group, and flock were considered a statistical population during analysis.
Estimation of diversity and identification of predominant taxa in each body site.
Alpha diversity index taxonomic richness (number of ASVs per sample) and Pielou’s evenness were calculated using the vegan package (99). Comparisons of value distributions between age groups were performed using a pairwise t test with Bonferroni correction for multiple comparisons. The dependence of richness and evenness on age was assessed with linear regression. The response of the two flock groups was measured separately for each body site. Comparisons between regressions from the same body site were performed using a modified t test described by the following functions:
| (1) |
| (2) |
where the slope (b), degrees of freedom (df), and coefficient of determination (R2) for the regression line were used to calculate the variance (v), which was subsequently used to calculate Student’s t.
For beta diversity analysis, we used unweighted UniFrac distances, calculated by applying the midpoint rooted tree to the feature table (R package GUniFrac [98]), to measure sample dispersion in composition space. Dimensionality was reduced using principal coordinate analysis (PCoA; R package vegan [99]), and differences between sample groups were quantified with pairwise permutational multivariate analysis of variance (PERMANOVA; R package pairwiseAdonis [101]). The distribution of all ASVs among body sites was visualized using a four-set Venn diagram (R package VennDiagram [100]).
Selection of predominant taxa was performed using a serial filtering scheme. The same filtering procedure was applied independently to each of the four body sites per flock (8 groups total), and the same parameters were used for each group. The procedure included several steps that consider age (6 groups/body site) as a subgrouping variable. The first filter (looking at rates of ASV occurrence) treated all subgroups independently, meaning that it filtered the data set 48 times (2 flocks, each with 4 body sites, each with 6 age groups) and pulled ASVs that were present in more than 50% of samples in each subgroup. From there, the second filter examined the results from each body site and retained those ASVs that emerged at each time point and persisted through the end of the study (16 weeks), from 1 to 16 weeks, from 3 to 16 weeks, from 5 to 16 weeks, from 8 to 16 weeks, and from 12 to 16 weeks. From each of these time series, the third filter simply reduces the number of resulting ASVs to those that exceed a threshold of relative abundance of 3.5% in at least one of the ages included in each series.
Data availability statement.
Raw data files and metadata are publicly available in the NCBI BioProject database under accession number PRJNA589216. Raw data files are also available through the Sequence Read Archive (SRA) under accession numbers SAMN13269218 through SAMN13269601. Pipe scripts for QIIME2 sequence processing and generation of the ASV table and R scripts for analysis are publicly available at https://github.com/kjmtaylor22/cwl-cats/tree/Taylor-et-al-2020---Respiratory-and-gut-microbiota-in-commercial-turkey-flocks.
Supplementary Material
ACKNOWLEDGMENTS
We thank the management team of the commercial farm participating in this study for providing turkeys and giving us access to flock treatment and management data related to this study. The identity of the commercial farm referenced in this study will remain anonymous as a condition of their open sharing of rearing metadata and cooperation during sampling.
We have no conflicts of interest to declare.
Sequence processing and analysis were performed using the resources of the Molecular & Cellular Imaging Center, Ohio Agricultural Research and Development Center, The Ohio State University.
This project was supported by Agriculture and Food Research Initiative competitive grant number 2015-68004-23131 from the USDA National Institute of Food and Agriculture (to C.-W.L. and T.J.J.).
C.-W.L. and T.J.J. developed the broad concept of the study. C.-W.L., J.M.N., and M.C.A. designed and managed the study. J.M.N., M.C.A., H.J., M.E., M.K.C., and C.-W.L. collected the samples. J.M.N., M.C.A., and A.G. performed sample processing and DNA extraction. B.P.W. and T.J.J. managed the DNA sequencing. K.J.M.T. and J.M.N. performed bioinformatics analyses. K.J.M.T. and J.M.N. wrote the manuscript with input from all authors. C.-W.L. provided reagents and sampling resources. All authors read and approved the final manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Jankowski J, Zduńczyk Z, Mikulski D, Przybylska-Gornowicz B, Sosnowska E, Juśkiewicz J. 2013. Effect of whole wheat feeding on gastrointestinal tract development and performance of growing turkeys. Anim Feed Sci Technol 185:150–159. doi: 10.1016/j.anifeedsci.2013.07.012. [DOI] [Google Scholar]
- 2.Lei K, Li YL, Yu DY, Rajput IR, Li WF. 2013. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci 92:2389–2395. doi: 10.3382/ps.2012-02686. [DOI] [PubMed] [Google Scholar]
- 3.Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter A-M, Verlhac V. 2017. Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol 234:88–100. doi: 10.1016/j.anifeedsci.2017.09.012. [DOI] [Google Scholar]
- 4.Choi KY, Lee TK, Sul WJ. 2015. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens–a review. Asian-Australas J Anim Sci 28:1217–1225. doi: 10.5713/ajas.15.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon K, Verwoolde MB, Zhang J, Smidt H, de Vries Reilingh G, Kemp B, Lammers A. 2016. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult Sci 95:1543–1554. doi: 10.3382/ps/pew088. [DOI] [PubMed] [Google Scholar]
- 6.Danzeisen JL, Calvert AJ, Noll SL, McComb B, Sherwood JS, Logue CM, Johnson TJ. 2013. Succession of the turkey gastrointestinal bacterial microbiome related to weight gain. PeerJ 1:e237. doi: 10.7717/peerj.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtoglu V, Kurtoglu F, Seker E, Coskun B, Balevi T, Polat ES. 2004. Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol. Food Additives Contaminants 21:817–823. doi: 10.1080/02652030310001639530. [DOI] [PubMed] [Google Scholar]
- 8.Pan D, Yu Z. 2014. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borda-Molina D, Seifert J, Camarinha-Silva A. 2018. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput Struct Biotechnol J 16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, Lee MD, Collett SR, Johnson TJ, Cox NA. 2014. The chicken gastrointestinal microbiome. FEMS Microbiol Lett 360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- 11.Scupham AJ. 2009. Campylobacter colonization of the turkey intestine in the context of microbial community development. Appl Environ Microbiol 75:3564–3571. doi: 10.1128/AEM.01409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scupham AJ. 2007. Succession in the intestinal microbiota of preadolescent turkeys. FEMS Microbiol Ecol 60:136–147. doi: 10.1111/j.1574-6941.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.D'Andreano S, Sànchez Bonastre A, Francino O, Cuscó Martí A, Lecchi C, Grilli G, Giovanardi D, Ceciliani F. 2017. Gastrointestinal microbial population of turkey (Meleagris gallopavo) affected by hemorrhagic enteritis virus. Poult Sci 96:3550–3558. doi: 10.3382/ps/pex139. [DOI] [PubMed] [Google Scholar]
- 14.Calvert AJ. 2012. Light turkey syndrome: field study and inoculation trial. MSc thesis. University of Minnesota, Minneapolis, MN. [Google Scholar]
- 15.Zduńczyk Z, Jankowski J, Kaczmarek S, Juśkiewicz J. 2015. Determinants and effects of postileal fermentation in broilers and turkeys part 1: gut microbiota composition and its modulation by feed additives. Worlds Poult Sci J 71:37–47. doi: 10.1017/S0043933915000045. [DOI] [Google Scholar]
- 16.Ward TL, Weber BP, Mendoza KM, Danzeisen JL, Llop K, Lang K, Clayton JB, Grace E, Brannon J, Radovic I, Beauclaire M, Heisel TJ, Knights D, Cardona C, Kogut M, Johnson C, Noll SL, Arsenault R, Reed KM, Johnson TJ. 2019. Antibiotics and host-tailored probiotics similarly modulate effects on the developing avian microbiome, mycobiome, and host gene expression. mBio 10:e02171-19. doi: 10.1128/mBio.02171-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danzeisen JL, Clayton JB, Huang H, Knights D, McComb B, Hayer SS, Johnson TJ. 2015. Temporal relationships exist between cecum, ileum, and litter bacterial microbiomes in a commercial turkey flock, and subtherapeutic penicillin treatment impacts ileum bacterial community establishment. Front Vet Sci 2:56. doi: 10.3389/fvets.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson TJ, Cowan AA, Vallin HE, Onime LA, Oyama LB, Cameron SJ, Gonot C, Moorby JM, Waddams K, Theobald VJ, Leemans D, Bowra S, Nixey C, Huws SA. 2017. Characterization of the microbiome along the gastrointestinal tract of growing turkeys. Front Microbiol 8:1089. doi: 10.3389/fmicb.2017.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niedermeyer JA, Ring L, Miller WG, Genger S, Lindsey CP, Osborne J, Kathariou S. 2018. Proximity to other commercial turkey farms affects colonization onset, genotypes, and antimicrobial resistance profiles of Campylobacter spp. in Turkeys: suggestive evidence from a paired-farm model. Appl Environ Microbiol 84:e01212-18. doi: 10.1128/AEM.01212-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AH, Rehberger TG. 2018. Bacteria and fungi in day-old turkeys vary among companies, collection periods, and breeder flocks. Poult Sci 97:1400–1411. doi: 10.3382/ps/pex429. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson EE, Stanley D, Hughes RJ, Moore RJ. 2017. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. PeerJ 5:e3587. doi: 10.7717/peerj.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hieke A-S, Hubert SM, Athrey G. 2019. Circadian disruption and divergent microbiota acquisition under extended photoperiod regimens in chicken. PeerJ 7:e6592. doi: 10.7717/peerj.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Lilburn M, Yu Z. 2016. Intestinal microbiota of broiler chickens as affected by litter management regimens. Front Microbiol 7:593. doi: 10.3389/fmicb.2016.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cressman MD, Yu Z, Nelson MC, Moeller SJ, Lilburn MS, Zerby HN. 2010. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl Environ Microbiol 76:6572–6582. doi: 10.1128/AEM.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ. 2011. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One 6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson TJ, Youmans BP, Noll S, Cardona C, Evans NP, Karnezos TP, Ngunjiri JM, Abundo MC, Lee C-W. 2018. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl Environ Microbiol 84:e00362-18. doi: 10.1128/AEM.00362-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngunjiri JM, Taylor KJM, Abundo MC, Jang H, Elaish M, KC M, Ghorbani A, Wijeratne S, Weber BP, Johnson TJ, Lee C-W. 2019. Farm stage, bird age, and body site dominantly affect the quantity, taxonomic composition, and dynamics of respiratory and gut microbiota of commercial layer chickens. Appl Environ Microbiol 85:e03137-18. doi: 10.1128/AEM.03137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naylor CJ, Al-Ankari AR, Al-Afaleq AI, Bradbury JM, Jones RC. 1992. Exacerbation of Mycoplasma gallisepticum infection in turkeys by rhinotracheitis virus. Avian Pathol 21:295–305. doi: 10.1080/03079459208418844. [DOI] [PubMed] [Google Scholar]
- 29.Power J, Jordan F. 1976. A comparison of the virulence of three strains of Mycoplasma gallisepticum and one strain of Mycoplasma gallinarum in chicks, turkey poults, tracheal organ cultures and embryonated fowl eggs. Res Vet Sci 21:41–46. doi: 10.1016/S0034-5288(18)33391-5. [DOI] [PubMed] [Google Scholar]
- 30.De Rosa M, Droual R, Chin RP, Shivaprasad HL, Walker RL. 1996. Ornithobacterium rhinotracheale infection in turkey breeders. Avian Dis 40:865–874. doi: 10.2307/1592311. [DOI] [PubMed] [Google Scholar]
- 31.Glendinning L, McLachlan G, Vervelde L. 2017. Age-related differences in the respiratory microbiota of chickens. PLoS One 12:e0188455. doi: 10.1371/journal.pone.0188455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shabbir MZ, Malys T, Ivanov YV, Park J, Shabbir MAB, Rabbani M, Yaqub T, Harvill ET. 2015. Microbial communities present in the lower respiratory tract of clinically healthy birds in Pakistan. Poult Sci 94:612–620. doi: 10.3382/ps/pev010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallapura G, Hernandez-Velasco X, Pumford N, Bielke L, Hargis BM, Tellez G. 2014. Evaluation of respiratory route as a viable portal of entry for Salmonella in poultry. Vet Med Res Rep 5:59–79. doi: 10.2147/VMRR.S62775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantin-Jackwood MJ, Day JM, Jackwood MW, Spackman E. 2008. Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis 52:235–244. doi: 10.1637/8174-111507-Reg.1. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson-Noel N. 2013. Mycoplasmosis, p 875–941. In Swayne DE. (ed), Diseases of poultry, 13th ed. John Wiley & Sons, Ames, IA. [Google Scholar]
- 36.Hybrid Turkeys. 2019. Performance goals: converter commercial males. Hendrix Genetics, Kitchener, Ontario, Canada: https://www.hybridturkeys.com/documents/373/PG_C_CS_E_L_KG_08_17.pdf. [Google Scholar]
- 37.Yavari CA, Ramírez AS, Nicholas RAJ, Radford AD, Darby AC, Bradbury JM. 2017. Mycoplasma tullyi sp. nov., isolated from penguins of the genus Spheniscus. Int J Syst Evol Microbiol 67:3692–3698. doi: 10.1099/ijsem.0.002052. [DOI] [PubMed] [Google Scholar]
- 38.Bradbury JM, Abdul-Wahab OMS, Yavari CA, Dupiellet J-P, Bove JM. 1993. Mycoplasma imitans sp. nov. is related to mycoplasma gallisepticum and found in birds. Int J Syst Evol Microbiol 43:721–728. doi: 10.1099/00207713-43-4-721. [DOI] [PubMed] [Google Scholar]
- 39.Abdul-Wahab OM, Ross G, Bradbury JM. 1996. Pathogenicity and cytadherence of Mycoplasma imitans in chicken and duck embryo tracheal organ cultures. Infect Immun 64:563–568. doi: 10.1128/IAI.64.2.563-568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glisson JR. 1998. Bacterial respiratory disease of poultry. Poult Sci 77:1139–1142. doi: 10.1093/ps/77.8.1139. [DOI] [PubMed] [Google Scholar]
- 41.Blackall PJ, Soriano-Vargas EV. 2013. Infectious coryza and related bacterial infections, p 859–873. In Swayne DE. (ed), Diseases of poultry, 13th ed. John Wiley & Sons, Ames, IA. [Google Scholar]
- 42.van Empel P, van den Bosch H, Goovaerts D, Storm P. 1996. Experimental infection in turkeys and chickens with Ornithobacterium rhinotracheale. Avian Dis 40:858–864. doi: 10.2307/1592310. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstein R, Götz F. 2013. What distinguishes highly pathogenic staphylococci from medium- and non-pathogenic? Curr Top Microbiol Immunol 358:33–89. doi: 10.1007/82_2012_286. [DOI] [PubMed] [Google Scholar]
- 44.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang R, Xiao P, She R, Han S, Chang L, Zheng L. 2013. Culturable airborne bacteria in outdoor poultry-slaughtering facility. Microbes Environ 28:251–256. doi: 10.1264/jsme2.me12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luyckx K, Van Coillie E, Dewulf J, Van Weyenberg S, Herman L, Zoons J, Vervaet E, Heyndrickx M, De Reu K. 2017. Identification and biocide susceptibility of dominant bacteria after cleaning and disinfection of broiler houses. Poult Sci 96:938–949. doi: 10.3382/ps/pew355. [DOI] [PubMed] [Google Scholar]
- 47.Sghaier H, Bouchami O, Desler C, Lazim H, Saidi M, Rasmussen LJ, Ben Hassen A. 2012. Analysis of the antimicrobial susceptibility of the ionizing radiation-resistant bacterium Deinococcus radiodurans: implications for bioremediation of radioactive waste. Ann Microbiol 62:493–500. doi: 10.1007/s13213-011-0281-y. [DOI] [Google Scholar]
- 48.Angelakis E, Raoult D. 2010. The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS One 5:e10463. doi: 10.1371/journal.pone.0010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torok VA, Ophel-Keller K, Loo M, Hughes RJ. 2008. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl Environ Microbiol 74:783–791. doi: 10.1128/AEM.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, Percy NJ, Ophel-Keller K. 2011. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol 77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomason CA, Mullen N, Belden LK, May M, Hawley DM. 2017. Resident microbiome disruption with antibiotics enhances virulence of a colonizing pathogen. Sci Rep 7:16177. doi: 10.1038/s41598-017-16393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CLT, Chung C-Y, Kuo C-H, Kuo T-F, Yang C-W, Yang W-C. 2016. Beneficial effect of bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. PLoS One 11:e0146141. doi: 10.1371/journal.pone.0146141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 69:6816–6824. doi: 10.1128/aem.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martynova-Van Kley MA, Oviedo-Ron EO, Dowd SE, Hume M, Nalian A. 2012. Effect of Eimeria infection on cecal microbiome of broilers fed essential oils. Int J Poultry Sci 11:747–755. doi: 10.3923/ijps.2012.747.755. [DOI] [Google Scholar]
- 55.Allen-Vercoe E, Strauss J, Chadee K. 2011. Fusobacterium nucleatum. Gut Microbes 2:294–298. doi: 10.4161/gmic.2.5.18603. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y-J, Wu H, Wu S-D, Lu N, Wang Y-T, Liu H-N, Dong L, Liu T-T, Shen X-Z. 2018. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J Gastroenterol Hepatol 33:1844–1852. doi: 10.1111/jgh.14281. [DOI] [PubMed] [Google Scholar]
- 57.Langworth BF. 1977. Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol Rev 41:373–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, DeVinney R, Lynch T, Allen-Vercoe E. 2011. Invasive potential of gut mucosa-derived fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis 17:1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 59.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. 2012. The application of ecological theory toward an understanding of the human microbiome. Science 336:1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huxley EJ, Viroslav J, Gray WR, Pierce AK. 1978. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med 64:564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 61.Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, Schmidt TM. 2015. Application of a neutral community model to assess structuring of the human lung microbiome. mBio 6:e02284-14. doi: 10.1128/mBio.02284-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. 2016. The microbiome and the respiratory tract. Annu Rev Physiol 78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Awad WA, Mann E, Dzieciol M, Hess C, Schmitz-Esser S, Wagner M, Hess M. 2016. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front Cell Infect Microbiol 6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J, Abe F, Osawa R. 2016. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumas MD, Polson SW, Ritter D, Ravel J, Gelb J, Morgan R, Wommack KE. 2011. Impacts of poultry house environment on poultry litter bacterial community composition. PLoS One 6:e24785. doi: 10.1371/journal.pone.0024785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oakley BB, Buhr RJ, Ritz CW, Kiepper BH, Berrang ME, Seal BS, Cox NA. 2014. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet Res 10:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Zhong H, Li Y, Shi Z, Zhang Z, Zhou X, Ren H, Tang S, Han X, Lin Y, Wang D, Yang F, Fang C, Fu Z, Wang L, Zhu S, Hou Y, Xu X, Yang H, Wang J, Kristiansen K, Li J, Ji L. 2019. Age-dependent sexual dimorphism in the adult human gut microbiota. bioRxiv doi: 10.1101/646620. [DOI]
- 69.Mahnic A, Rupnik M. 2018. Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS One 13:e0209209. doi: 10.1371/journal.pone.0209209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waite DW, Taylor MW. 2014. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front Microbiol 5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K-C, Kil DY, Sul WJ. 2017. Cecal microbiome divergence of broiler chickens by sex and body weight. J Microbiol 55:939–945. doi: 10.1007/s12275-017-7202-0. [DOI] [PubMed] [Google Scholar]
- 72.Kers JG, Velkers FC, Fischer EAJ, Hermes GDA, Stegeman JA, Smidt H. 2018. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol 9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller ET, Svanback R, Bohannan B. 2018. Microbiomes as metacommunities: understanding host-associated microbes through metacommunity ecology. Trends Ecol Evol 33:926–935. doi: 10.1016/j.tree.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL. 2016. Analysis of the microbiome: advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun 469:967–977. doi: 10.1016/j.bbrc.2015.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park SH, Kim SA, Rubinelli PM, Roto SM, Ricke SC. 2017. Microbial compositional changes in broiler chicken cecal contents from birds challenged with different Salmonella vaccine candidate strains. Vaccine 35:3204–3208. doi: 10.1016/j.vaccine.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 77.Tarabichi Y, Li K, Hu S, Nguyen C, Wang X, Elashoff D, Saira K, Frank B, Bihan M, Ghedin E, Methé BA, Deng JC. 2015. The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome 3:74. doi: 10.1186/s40168-015-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Gordon JI. 2008. An invitation to the marriage of metagenomics and metabolomics. Cell 134:708–713. doi: 10.1016/j.cell.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 79.Bushnell B, Rood J, Singer E. 2017. BBMerge–accurate paired shotgun read merging via overlap. PLoS One 12:e0185056. doi: 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 81.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Caporaso JG. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer K-H, Whitman WB, Euzéby J, Amann R, Rosselló-Móra R. 2014. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol 12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 87.R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 88.Morgan M. 2019. BiocManager: access the bioconductor project package repository. R package version 1.30.10 https://CRAN.R-project.org/package=BiocManager.
- 89.McMurdie PJ, Paulson JN. 2019. biomformat: an interface package for the BIOM file format. https://github.com/joey711/biomformat/.
- 90.Wickham H, Francois R, Henry L, Muller K. 2019. dplyr: a grammar of data manipulation. https://dplyr.tidyverse.org.
- 91.Wickham H. 2007. Reshaping data with the reshape package. J Stat Soft 21:1–20. doi: 10.18637/jss.v021.i12. [DOI] [Google Scholar]
- 92.Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 93.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
- 94.Auguie B. 2017. gridExtra: miscellaneous functions for “grid” graphics. R package version 2.3. https://CRAN.R-project.org/package=gridExtra.
- 95.Neuwirth E. 2014. RColorBrewer: ColorBrewer palettes. R package version 1.1–2. https://CRAN.R-project.org/package=RColorBrewer.
- 96.Kassambara A. 2020. ggpubr: “ggplot2” based publication ready plots. R package version 0.2.5. https://CRAN.R-project.org/package=ggpubr.
- 97.Dragulescu AA, Arendt C. 2020. xlsx: read, write, format Excel 2007 and Excel 97/2000/XP/2003 files. R package version 0.6.3. https://CRAN.R-project.org/package=xlsx.
- 98.Chen J. 2018. GUniFrac: generalized UniFrac distances. R package version 1.1. https://CRAN.R-project.org/package=GUniFrac.
- 99.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2019. vegan: community ecology package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan.
- 100.Chen H. 2018. VennDiagram: generate high-resolution Venn and Euler plots. R package version 1.6.20. https://CRAN.R-project.org/package=VennDiagram.
- 101.Martinez AP. 2017. pairwiseAdonis: pairwise multilevel comparison using Adonis. R package version 0.0.1. https://github.com/pmartinezarbizu/pairwiseAdonis.
- 102.Li I-C, Wu S-Y, Liou J, Liu H-H, Chen J, Chen C-C. 2018. Effects of Deinococcus spp. supplement on egg quality traits in laying hens. Poult Sci 97:319–327. doi: 10.3382/ps/pex281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data files and metadata are publicly available in the NCBI BioProject database under accession number PRJNA589216. Raw data files are also available through the Sequence Read Archive (SRA) under accession numbers SAMN13269218 through SAMN13269601. Pipe scripts for QIIME2 sequence processing and generation of the ASV table and R scripts for analysis are publicly available at https://github.com/kjmtaylor22/cwl-cats/tree/Taylor-et-al-2020---Respiratory-and-gut-microbiota-in-commercial-turkey-flocks.