In this study, we developed a novel strategy using indigenous internal standards to normalize the high-throughput amplicon sequencing results. We chose two Lactobacillus species as indigenous internal standards and characterized the absolute abundance of the bacterial community. Further, we identified Lactobacillus as the key flavor producer using quantitative microbiome profiling combined with multivariate statistics and metatranscriptomic analysis. This work developed a novel strategy for absolute quantitative abundance analysis of microbiota and expanded our understanding of the role of Lactobacillus in food fermentation.
KEYWORDS: Chinese liquor, indigenous internal standards, key flavor producer, microbiota, quantitative microbiome profiling
ABSTRACT
Identifying the functional microbes in spontaneous food fermentation is important for improving food quality. To identify the key flavor producers in Chinese liquor fermentation, we propose a novel quantitative microbiome profiling method that uses indigenous internal standards to normalize high-throughput amplicon sequencing results. We screened Lactobacillus acetotolerans and Lactobacillus jinshani as indigenous internal standards based on their high distribution frequencies and relative abundances. After determining the absolute abundance of indigenous internal standards using quantitative PCR with species-specific primers, the liquor-fermented bacterial community and its dynamics were better characterized by internal standards normalization. Based on quantitative microbiome profiling, we identified that Lactobacillus was a key flavor producer correlated with eight flavor compounds. Metatranscriptomic analysis indicated that Lactobacillus was active in transcribing genes involving the biosynthesis of flavor compounds and their precursors. This work has developed a novel and extensible absolute quantification method for microbiota that will alleviate concerns in the statistical analyses based on relative microbiome profiling, and shed insights into the function of Lactobacillus in food fermentation. It can potentially be applied to other microbial ecology studies.
IMPORTANCE In this study, we developed a novel strategy using indigenous internal standards to normalize the high-throughput amplicon sequencing results. We chose two Lactobacillus species as indigenous internal standards and characterized the absolute abundance of the bacterial community. Further, we identified Lactobacillus as the key flavor producer using quantitative microbiome profiling combined with multivariate statistics and metatranscriptomic analysis. This work developed a novel strategy for absolute quantitative abundance analysis of microbiota and expanded our understanding of the role of Lactobacillus in food fermentation.
INTRODUCTION
Fermented foods such as cheese (1), vinegar (2), and dairy products (3) always require a multispecies community. Uncovering the functional microbes is a critical topic for improving food quality. Chinese liquor is one of six distilled spirits in the world with thousands of years of history (4). It is produced by a representative spontaneous fermentation involving complex microbiota (5, 6). Microbiota produce various flavor compounds via transforming sugars and amino acids. However, the key flavor producer in the bacterial community is unclear.
Quantitative microbiome profiling (quantified microbiota taxa by absolute abundance) is essential for microbiota studies, including microbial interactions (7, 8), the relationship of microbiota with environmental variables (9), and metabolic outputs (8, 10, 11). The development of high-throughput amplicon sequencing technology provides novel insights into the microbial community profiles in the Chinese liquor fermentation at high taxonomic resolution (12–14). However, this method allows only relative microbiome profiling (quantified microbiota taxa by relative abundance), which may deliver a skewed pattern of the actual microbial community dynamics (7, 8, 15, 16) and lead to difficulties in the subsequent statistical analyses (17, 18). As a result, quantitative microbiome profiling is urgently needed for the identification of flavor producers in liquor fermentation.
Currently, amplicon sequencing combined with added exogenous internal standards spiked into the sample has become a useful tool to absolutely evaluate the variation of bacterial abundance. For example, Stämmler et al. (19) took Salinibacter ruber, Rhizobium radiobacter, and Alicyclobacillus acidiphilus as exogenous internal standards and added whole cells to samples to accurately estimate the absolute bacterial abundances in the gut samples. Tourlousse et al. (20) added synthetic 16S rRNA genes as the internal standards to estimate the absolute microbial abundances in the soil samples. Quantitative microbiome profiling can be achieved by manually adding internal standards. However, there are still challenges in the actual application. First, it is difficult to predict the presence of microorganisms in the uncharacterized microbiota system, making it hard to select the species to use as the internal standards. Meanwhile, although there are commercial solutions such as Zymo for certain systems, which provide alternative exogenous internal standards, these consume sequencing capacity and may result in failure if the spike-in standards are incorrectly loaded. For example, when the added concentration was more than 1.23 × 107 cells/g, the analyzed microbiota community structure was different from the original microbiota (16). Due to the dispersive amounts of members in the different microbiota, it is hard to choose the suitable addition concentrations of the internal standards. Hence, it is necessary to build a convenient and efficient quantitative method independent of exogenous internal standards.
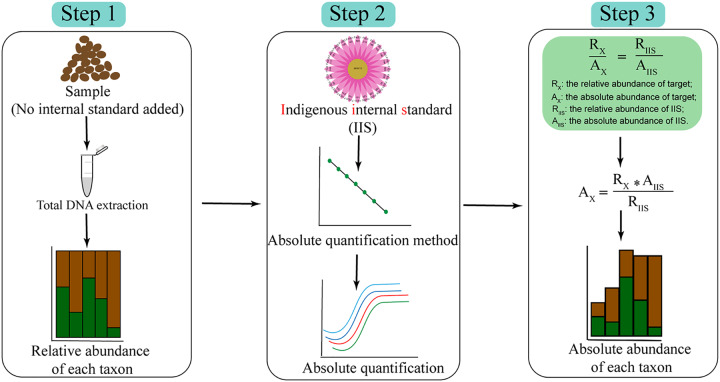
In this work, to identify the key flavor producer(s) during Chinese liquor fermentation, we developed a novel absolute quantification strategy using indigenous internal standards to normalize the high-throughput amplicon sequencing results. The workflow is shown in Fig. 1. Briefly, the absolute and relative abundance of indigenous internal standards (AIIS and RIIS) were determined using species-specific quantitative PCR (qPCR) and high-throughput amplicon sequencing. The multiple relationships between relative and absolute abundances of indigenous internal standards allowed the absolute abundance of total bacteria for each genus to be calculated based on the relative abundances. Further, we identified a key flavor producer in the bacterial community using absolute quantitative results. The metabolic activity of the flavor producer was verified via metatranscriptome analysis. This work developed a novel strategy for quantitative microbiome profiling and shed new light on the function of Lactobacillus in food fermentations.
FIG 1.
The workflow of absolute quantification method for microbiota. This method consists of 3 steps. Step 1: high-throughput amplicon sequencing of each sample. Step 2: screening and quantifying the indigenous internal standards. Step 3: normalizing the results of high-throughput amplicon sequencing with indigenous internal standards.
RESULTS
The quantitative microbiome profiling method based on indigenous internal standards.
16S rRNA amplicon sequencing (V3-V4 region) was applied to examine microbial composition during the liquor fermentation. The raw sequencing data from amplicon sequencing are summarized in Table S1 in the supplemental material. We screened indigenous internal standards based on distribution frequency and relative abundance. For the distribution frequency, 10 core species were shared in all detected samples (Fig. 2A). As shown in the phylogenetic tree (Fig. 2B), they belonged to five genera, including Lactobacillus, Pediococcus, Weissella, Leuconostoc, and Bacillus. The relative abundances of 10 species are shown in Fig. 2C. Among them, Lactobacillus jinshani and Lactobacillus acetotolerans were considered candidate indigenous internal standards due to their higher relative abundances than others (average relative abundance of >10%, minimum relative abundance of >0.1%).
FIG 2.
Screening indigenous internal standards. (A) The core species (shared in all samples). (B) Phylogenetic placement of the core species. The maximum-likelihood phylogenetic tree was constructed using the alignment of the 16S rRNA V3-V4 region. (C) The relative abundance of core species during the fermentation. Min, Ave, and Max represent minimum, average, and maximum relative abundance during the fermentation, respectively.
We chose a qPCR method to quantify indigenous internal standards in the microbiome samples. The brief details of species-specific primers PLace and PLjin for L. acetotolerans and L. jinshani, respectively, are shown in Table 1. The primers did not match to other nontarget organisms in the NCBI NR database. The PLace primers were designed based on a species-specific gene (one helix-turn-helix transcriptional regulator gene, GenBank BAQ57713.1). PLace exhibited an exclusivity of 100% for 21 nontarget organisms from liquor fermentation (Fig. S1A). We next developed the qPCR method using PLace. The standard curve was obtained by calculating linearity between logarithm of the number of copies per reaction and Ct value. The correlation was linear (R2 = 0.99) and ranged from 10.7 to 1.07 × 108 copies per reaction, suggesting that this qPCR method could be used to quantify the number of L. acetotolerans present in the samples. PLjin primers were designed based on the 16S rRNA gene (GenBank KU674948.1) because there were base sequence differences at the same region with other close species (21). PLjin had a 100% exclusivity (Fig. S1B). The logarithm of the number of copies ranging from 17.8 to 17.8 × 108 copies per reaction displayed a linear correlation with corresponding Ct value (R2 = 0.99) which had been reported in our previous study (21) (the Lactobacillus sp. in that study was subsequently found to be a strain of the recently described L. jinshani [22]). Meanwhile, we also developed qPCR assays for the other three core species, Pediococcus pentosaceus, Weissella paramesenteroides, and Bacillus coagulans. As shown in Fig. S2, PPpen, PWpar, and PBcoa (the sets of species-specific primers for P. pentosaceus, W. paramesenteroides, and B. coagulans, respectively) all showed 100% exclusivity. The standard curves had strong linear correlation (R2 = 0.99) and ranged from 28.8 to 2.88 × 108 copies per reaction for PPpen, 17 to 1.70 × 108 copies per reaction for PWpar, and 10.7 to 1.07 × 108 copies per reaction for PBcoa (Table 1).
TABLE 1.
Species-specific primers used in this study
| Primer name | Primer sequence (5′–3′) | Species | GenBank ID | Standard curvea | Amplicon length | Reference or source |
|---|---|---|---|---|---|---|
| PLace F | AAAAAGCAGAGTGGAGAAAATACT | L. acetotolerans | BAQ57713.1 | y = −3.65x + 34.67; R2 = 0.99; 10.7–1.07 × 108 (copies/reaction) | 119 bp | This study |
| PLace R | CCAATAAAAAGAGCAACAGCA | |||||
| PLjin F | CGCACTCCCGTAGATGATTTTGA | L. jinshani | KU674948.1 | y = −3.54x + 33.85; R2 = 0.99; 17.8–17.8 × 108 (copies/reaction) | 445 bp | (21) |
| PLjin R | TCACTACCAAGCCATTTCCTAC | |||||
| PPpen F | CTATTGACTTGGTCGTTATTGATTCC | P. pentosaceus | WP_002833728 | y = −3.14x + 33.33; R2 = 0.99; 28.8–2.88 × 108 (copies/reaction) | 72 bp | (41) |
| PPpen R | CCCCCATCTCTCCATCAATTT | |||||
| PWpar F | CTAGAGGCGGCGAAGTCAGT | W. paramesenteroides | ATF40921.1 | y = −3.44x + 36.79; R2 = 0.99; 17–1.70 × 108 (copies/reaction) | 101 bp | This study |
| PWpar R | CTATTCGCGTCGCCAACCAT | |||||
| PBcoa F | CTCACGGAAGAGCAAGCTTG | B. coagulans | QAU28402.1 | y = −3.23x + 36.74; R2 = 0.99; 10.7–1.07 × 108 (copies/reaction) | 293 bp | (42) |
| PBcoa R | GTTTCTGAAATGTATGCACG |
x is the Ct value; y is the log10 of the number of gene copies per reaction. The amplification conditions of PLace, PPpen, PWpar, and PBcoa were 95°C for 5 min, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. The amplification conditions of PLjin were 98°C for 1 min, followed by 40 cycles of 98°C for 10 s, 55°C for 30 s and 72°C for 25 s. Species-specific gene (GenBank BAQ57713.1) for L. acetotolerans was screened from genome sequence GenBank AP014808.1. Species-specific gene (GenBank ATF40921.1) for W. paramesenteroides was screened from genome sequence GenBank: CP023501.1. The species-specific primers PLace and PWpar were designed using Batchprimer 3 (40) with the following parameters: amplicon size, 100 to 300 bp; amplicon GC content, 40 to 60% (optimal 50%); primer length, 18 to 28 nt (optimal 20 nt); and melting temperature (Tm) 57 to 63°C (optimal 60°C, maximum difference per primer pair <3°C).
We collected 48 fermented grain samples from 12 provinces across China to reveal spatial distributions of the two indigenous internal standards L. acetotolerans and L. jinshani (Fig. S3). The absolute abundance data of L. jinshani in different samples were collected from a previous study (21). L. acetotolerans was detected in all samples with concentrations ranging from 4.71 ± 0.16 to 7.28 ± 0.05 log10 (copies/g). L. jinshani was detected in 36 samples from 9 provinces with the concentrations ranging from 3.67 ± 1.01 to 7.27 ± 0.04 log10 (copies/g). This result demonstrated that these two indigenous internal standards could be widely applied in different liquor fermentation systems.
Quantitative microbiome profiling during liquor fermentation.
Relative bacterial abundance during liquor fermentation is shown in Fig. 3A. Eight genera were detected at a relative abundance of >1%, including Lactobacillus, Weissella, Leuconostoc, Pediococcus, Acetobacter, Bacillus, Pseudoxanthomonas, and Sphingobacterium.
FIG 3.
Dynamic variation of microbiota during fermentation. (A) The relative abundance of microbial genera during fermentation. The genera with average relative abundances of <1% were combined as “others.” (B) The absolute abundances of internal standards L. acetotolerans and L. jinshani. (C) The total bacterial absolute abundance calculated by internal standard L. acetotolerans. (D) The difference between absolute abundances of Weissella calculated with L. acetotolerans and L. jinshani as internal standards, where K is slope.
Absolute growth dynamics of indigenous internal standards (L. acetotolerans and L. jinshani) are shown in Fig. 3B. The absolute abundance of bacterial community was then estimated via normalizing high-throughput amplicon sequencing results with the internal standard (L. acetotolerans), as described in Figure 1. There was a clear growth trend during fermentation which could be divided into two stages: stage I (day 0 to 15) and II (day 15 to 45) (Fig. 3C). During stage I, the absolute abundance of bacteria gradually increased from 5.37 to 7.80 log10 (copies/g). During stage II, the absolute abundance of bacteria slowly decreased to 7.47 log10 (copies/g).
To validate the consistency between quantitative results of using different internal standards, the absolute abundance of Weissella was calculated using L. acetotolerans and L. jinshani, respectively. They were significantly correlated (Pearson’s r = 0.98, slope K = 1.01, P = 0.001) (Fig. 3D), and there was no significant difference (P = 0.87) between quantitated results. We found similar results (except for low relative abundance data < 0.1%) when using the other three core species, P. pentosaceus, W. paramesenteroides, and B. coagulans, as internal standards, all of which are distantly related to Lactobacillus (Fig. S4). These results suggested that calculating bacterial absolute abundances from different internal standards (with high relative abundances of >0.1%) were consistent.
In addition, we extracted the data belonging to 10 core species from amplicon sequencing data and metagenomic sequencing data to compare their relative proportions. As shown in Fig. S5, there were significant correlations (Pearson’s r > 0.99, P < 0.001) and no significant differences (P = 0.55, day 8; P = 0.09, day 15; P = 0.81, day 40) between amplicon sequencing and metagenomic sequencing results. After removing two dominant species (L. acetotolerans and L. jinshani), there were still significant correlations for the rest of the core species (Pearson’s r = 0.95, P < 0.001, day 8; Pearson’s r = 0.90, P = 0.002, day 15; Pearson’s r = 0.93, P < 0.001, day 40).
Further, the differences between the relative and absolute abundance of each dominant genus were analyzed (Fig. S6). Except Lactobacillus (Pearson’s r = 0.94, P < 0.001), the other seven genera were not significantly correlated (Pearson’s r < 0.6 or P > 0.05).
Uncovering a key flavor producer based on quantitative microbiome profiling.
In order to reveal the key flavor producer during liquor fermentation, a total of 20 key flavor compounds were identified, including three alcohols, two aldehydes, five acids, nine esters and one phenol (Fig. 4A). Two genera were significantly correlated (Pearson’s r > 0.6, P < 0.05) with a total of eight flavor compounds (Fig. 4B). Among them, Pediococcus was significantly correlated with one flavor compound, while Lactobacillus was significantly correlated with eight flavor compounds, including seven esters (ethyl phenylacetate, ethyl octanoate, 2-phenylethyl acetate, ethyl 2-methyl butanoate, ethyl 3-phenylpropanoate, ethyl hexanoate, and ethyl butanoate) and one acid (phenylacetic acid).
FIG 4.
Relationship between microbial genera and key flavor compounds. (A) Heat map of key flavor compounds in the fermentation. A total of 20 key compounds were detected during fermentation. The Z-score was used for data standardization. (B) Correlation network between microbial genera and flavor compounds based on quantitative microbiome profiling, where lines indicates a significant positive correlation (Pearson’s r > 0.6, P < 0.05).
Validating the role of Lactobacillus during liquor fermentation with metatranscriptomic analysis.
In order to verify metabolically active microbes during fermentation, we employed a metatranscriptome approach at a growth time point (day 8) and a stable time point (day 15) (Fig. 3C). The raw sequencing data of metatranscriptome are summarized in Table S2. Twenty-nine bacterial genera were transcribed during fermentation (Fig. S7). Among them, Lactobacillus possessed the highest relative transcript abundance across both time points (day 8, 92.94 ± 1.56%; day 15, 98.42 ± 0.09%). This result suggested that Lactobacillus was the dominant metabolically active microbe during liquor fermentation.
To validate the flavor production activity of Lactobacillus, we investigated the transcription characteristics of genes involved in the biosynthesis of the eight related flavor compounds (Fig. 5). Lactobacillus was active in transcribing genes associated with the biosynthesis of flavor compound precursors. For example, the bacterium transcribed the gene encoding phenylacetyl-CoA thioesterase (day 8, 85.76 ± 33.32 fragments per kilobase of transcript per million fragments mapped [FPKM]; day 15, 189.18 ± 42.24 FPKM) involved in the synthesis of phenylacetic acid (23) (the precursor of ethyl phenylacetate). Other transcribed genes encoded aryl-alcohol dehydrogenase (day 8, 6029.93 ± 1816.37 FPKM; day 15, 15590.99 ± 3872.91 FPKM) catalyzing the synthesis of 2-phenyl ethanol (the precursor of 2-phenylethyl acetate) and acyl-ACP thioesterase (day 8, 465.73 ± 37.29 FPKM; day 15, 653.08 ± 47.12 FPKM) catalyzing the synthesis of octanoic acid (the precursor of ethyl octanoate). It also transcribed the gene encoding acetate kinase (day 8, 323.02 ± 38.47 FPKM; day 15, 260.34 ± 32.16 FPKM) involved in the synthesis of acetic acid, the precursor of ethyl esters. In addition, most esters can be formed by the enzymatic condensation of acids and alcohols. Lactobacillus showed high transcript abundance for the gene encoding aryl esterase (day 8, 354.67 ± 39.12 FPKM; day 15, 465.10 ± 59.79 FPKM), which catalyzes the synthesis of esters (23). This result confirms that Lactobacillus plays an important role in flavor formation during liquor fermentation.
FIG 5.
Metabolic capacity of Lactobacillus associated with the formation of eight flavor compounds and their precursors. Heat map shows the gene transcription level; color depth represents the gene expression levels.
DISCUSSION
Quantitative microbiome profiling is essential for study of the function of the microbiota (24). To identify the key flavor producer during liquor fermentation, this study utilized indigenous species (L. acetotolerans and L. jinshani) as internal standards to construct an absolute quantification method for microbiota. Compared with methods utilizing exogenous internal standards, this method avoids the need to select exogenous standards and then optimize their concentrations (Table S3).
This absolute quantification method for microbiota offers scalability in the Chinese liquor fermentation system, as L. acetotolerans was reported as the dominant species in liquor fermentation (13, 14, 25). In line with previous work, 48 fermented grain samples from representative liquor-making factories in 12 provinces were used to detect the distribution of L. acetotolerans. The species was widely distributed in different liquor fermentation systems across China (Fig. S3). In addition, L. jinshani was also widely distributed in liquor fermentation systems. The wide distribution of these indigenous species allows this method to be used in different liquor fermentation systems.
Besides the liquor fermentation system, core species were detected in different ecosystems. For example, one Lactobacillus species showed a ubiquitous presence during Spanish-style green olive fermentation (26). Three Lactobacillus species were present in each examined sample of fermented vegetables (27). Ten Prevotella species were detected in all 1,016 dairy cow rumen samples across four European countries (28). These reports indicate that our quantitative microbiome profiling method should have application across other microbial ecosystems. In addition, we proved the absolute abundances of bacteria calculated using different internal standards were consistent. This result allows us to screen different species as indigenous internal standards for different sample groups if there are no common species in all samples because of sample diversity. For example, no single species was shared between 1,186 samples from global wastewater treatment plants, but Macellibacteroides fermentans was detected in all 11 wastewater treatment plants sampled from South America (29). Thus, this species could be a candidate for use as the indigenous internal standard for the corresponding sample group. Further, identifying core species and developing qPCR assays for these species in different environments could be an effective way to reduce the work of this method and make it more practical. The environment-specific qPCR assays might also be useful to other studies without having to design new primers due to the high prevalence of core species in same-environment samples.
Amplicon sequencing combined with domain-level qPCR assays can also be used to evaluate variation in absolute abundance among taxa, such as domain-level bacterial qPCR assays (30). However, there was a contradiction in the selection of strand sequences when developing the standard curve, leading to a significant difference in results using different strand sequences (Fig. S8). Species-level qPCR assays will avoid the effect of differences in the amplification products or nucleotide degeneracies in the primer sequences. In addition, species-specific primers combined with TaqMan-hydrolysis probes can be used to detect single nucleotide differences. Species-level qPCR assays provide the possibility of using a single amplicon sequence variant (ASV) as an internal standard, which offers high specificity and intolerance of mismatches, as well as much easier optimization, relative to SYBR assays. Meanwhile, there may be additional confounding biases due to incomplete coverage and/or a different amplification bias between domain-level qPCR assays and domain-level next-generation sequencing amplicon assays. Therefore, a species-level assay combined with amplicon sequencing provides a better way to achieve quantitative microbiome profiling.
PCR bias is ubiquitous and unavoidable in the amplicon sequencing experiments. In this study, we used high template concentrations (15 ng/reaction) and minimized PCR cycle numbers (to 25 cycles) to reduce the PCR bias (31). In addition, we compared amplicon sequencing and metagenomic sequencing data and found there was no significant difference. This suggested that the potential PCR bias did not significantly affect the correlation analysis in this study, which was in line with a previous study (32). On the other hand, to further reduce the PCR bias in the future work, improving the high-throughput sequencing process by developing more efficient reagents, selecting more suitable primer pairs, or by developing new methods should be a goal.
Because relative abundance methods might provide a skewed outcome, more microbial ecologists have preferred to study microbiota profiles and functions using measures of absolute abundance (15, 20, 33). For example, Props et al. (15) found that microbial community succession was more well defined in the engineered freshwater ecosystem with absolute abundance profiling than relative abundance profiling, and Vandeputte et al. (8) revealed that Prevotella was associated with Crohn’s disease with absolute quantitative data. In line with previous work, we demonstrated significant differences between results obtained through absolute and relative abundance data. For example, Weissella might be considered an important flavor producer because it was correlated with three flavor compounds based on relative abundance data (Fig. S9). However, it was not correlated with any flavor compound using absolute abundance data, and metatranscriptomic analysis further confirmed this result. Thus, the differences between relative and absolute abundance results can be magnified and lead to skewed conclusions in subsequent statistical analysis, which has been a concern voiced by microbial ecologists (17, 18). Therefore, quantitative microbiome profiling should be applied to microbiome studies.
Lactobacillus was considered the lactic acid producer in Chinese liquor fermentation with metetrascriptomic analysis in previous work (34), but the metabolic activity related to flavor compounds was still unclear. Lactobacillus is a dominant genus within the lactic acid bacteria (LAB) and is important for flavor production in fermented foods (35, 36). For example, Lactobacillus is involved in the production of acetoin, acetic acid, 2-acetolactate, and phenylethanol in vinegar fermentation based on metagenomics analysis (37), is associated with the formation of esters in sourdough by stimulating fermentation (38), and contributes to lactate and acetate production during kimchi fermentation as determined by metatranscriptomic analysis (39). In line with previous work, we found that Lactobacillus was involved in the formation of various flavor compounds and their precursors, including phenyl acetic acid, 2-phenyl ethanol, octanoic acid, and acetate acid, within liquor fermentation. Esters are key flavor compounds in Chinese liquor. It has been generally accepted that esters are mainly produced by yeasts and molds in Chinese liquor fermentation. This work showed that, in contrast, the bacterium Lactobacillus was active in transcribing the gene encoding aryl esterase, associated with ester formation (23). The work sheds new light on the function of Lactobacillus in liquor fermentation.
In conclusion, this work has developed a novel strategy utilizing indigenous internal standards for quantitative microbiome profiling. Further, we uncovered and verified the in situ key flavor producer in liquor fermentation. The method developed in this study may be applicable for the functional analysis of the microbiota in the study of microbial ecosystems.
MATERIALS AND METHODS
Sample collection.
For most analyses, the fermented grain samples were collected from a liquor distillery in Weifang, Shandong, China, in December 2017. Samples were collected at day 0, 5, 8, 15, 20, 40, and 45 with three replicates, and stored at –20°C for DNA extraction and flavor compound determination. In addition, samples at day 8 and 15 were immediately frozen in liquid nitrogen after sampling and stored at –80°C for RNA extraction.
In the analysis of spatial distribution of indigenous internal standards, 48 fermented grain samples were collected from 12 provinces (4 samples were collected in one representational liquor distillery from each province). Samples were collected at the end of the fermentation and were stored at –20°C for DNA extraction. The detailed information on these samples is provided in Table S4.
Strains and growth conditions.
A total of 27 different strains were used in this study (Table 2). Lactic acid bacteria were statically cultured in MRS broth at 30°C for 3 days. Yeasts were cultured in yeast extract-peptone-dextrose (YPD) liquid medium at 200 rpm, 30°C for 1 day. Molds were cultured on peptone-dextrose-agar (PDA) plates at 30°C for 3 days. Bacillus, Escherichia, and Enterococcus spp. were cultured in LB liquid medium at 200 rpm, 37°C for 1 day.
TABLE 2.
Strains used in this study
| Strain | Sourcea |
|---|---|
| Lactobacillus acetotolerans CGMCC No. 14086 | CGMCC |
| Lactobacillus buchneri CGMCC No. 14271 | CGMCC |
| Bacillus licheniformis CGMCC No. 3962 | CGMCC |
| Zygosaccharomyces bailii CGMCC No. 4745 | CGMCC |
| Pichia kudriavzevii CGMCC No. 12418 | CGMCC |
| Saccharomycopsis fibuligera CGMCC No. 4742 | CGMCC |
| Aspergillus tubingensis CGMCC No. 8899 | CGMCC |
| Schizosaccharomyces pombe CGMCC No. 4744 | CGMCC |
| Lactobacillus brevis LBM 10003 | LBMAE |
| Lactobacillus crustorum LBM 10004 | LBMAE |
| Lactobacillus plantarum LBM 10005 | LBMAE |
| Lactobacillus curvatus LBM 10006 | LBMAE |
| Lactobacillus delbrueckii LBM 10007 | LBMAE |
| Lactobacillus murinus LBM 10008 | LBMAE |
| Lactobacillus reuteri LBM 10009 | LBMAE |
| Lactobacillus johnsonii LBM 10010 | LBMAE |
| Lactobacillus casei LBM 10011 | LBMAE |
| Weissella viridescens LBM 10012 | LBMAE |
| Weissella confusa LBM 10013 | LBMAE |
| Mucor rouxianus LBM 30001 | LBMAE |
| Pediococcus pentosaceus LBM 10014 | LBMAE |
| Weissella paramesenteroides LBM 10015 | LBMAE |
| Bacillus coagulans LBM 10016 | LBMAE |
| Escherichia coli LBM 10017 | LBMAE |
| Enterococcus lactis LBM 10018 | LBMAE |
| Bacillus amyloliquefaciens DSM7 | DSMZ |
| Saccharomyces cerevisiae CCTCC M2014463 | CTCC |
CGMCC, China General Microbiological Culture Collection Center; LBMAE, Lab of Brewing Microbiology and Applied Enzymology in Jiangnan University (Escherichia coli LBM 10017 was purchased from Beijing Solarbio Science & Technology Co., Ltd., Beijing, China, while the other strains were isolated from fermented grains; all strains are available to the public for free); DSMZ, German Collection of Microorganisms and Cell Cultures; CTCC, China Center for Type Culture Collection.
Total DNA and RNA extraction.
Genomic DNA of each strain was extracted using the genomic DNA extraction kit (Tiangen, Beijing, China) following the manufacturer’s protocol. The total DNA of fermented grains was extracted from 7 g of sample using the phenol chloroform method as previously described (34). The total RNA of fermented grains was extracted using the OMEGA soil RNA kit (Omega Bio-Tek, Norcross, GA) following the manufacturer’s protocol. Residual DNA was removed using RNase-free DNase (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Total DNA and RNA were quantified in a NanoDrop ND-1000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The quality of DNA and RNA were determined by agarose gel electrophoresis.
Quantitation methods for indigenous internal standards.
(i) Design and validation of species-specific primers. The genome sequences of L. acetotolerans (GenBank NZ_AP014808) and W. paramesenteroides (GenBank CP023501.1) were accessed at the National Center for Biotechnology Information (NCBI) database. The helix-turn-helix transcriptional regulator gene (GenBank BAQ57713.1) and the YjzC family gene (GenBank: ATF40921.1) were screened as species-specific genes for L. acetotolerans and W. paramesenteroides from genomic sequences using the BLAST program. The species-specific primer sets PLace for L. acetotolerans and PWpar for W. paramesenteroides were designed using Batchprimer3 (40) with the following parameters: amplicon size, 100 to 300 bp; amplicon GC content, 40 to 60% (optimal 50%); primer length, 18 to 28 nt (optimal 20 nt); and melting temperature (Tm) 57 to 63°C (optimal 60°C, maximum difference per primer pair <3°C). The species-specific primer set PLjin for L. jinshani was designed based on the16S rRNA gene (GenBank KU674948.1) as previously described (21). The species-specific primer set PPpen for P. pentosaceus was designed based on the recA gene (GenBank WP_002833728) as previously described (41). The species-specific primer set PBcoa for B. coagulans was designed based on the ComK gene (GenBank QAU28402.1) as previously described (42).
We used two methods to validate the specificity of each primer. First, we used the Primer-blast algorithm (43) to simulate PCR, taking the NCBI NR database as the matching target (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Second, we used cross-PCR, taking the genomic DNA of the target organism and nontarget organisms as the PCR template, respectively. For PLjin, because we did not get a pure culture of L. jinshani, a synthetic 16S rRNA gene of L. jinshani HSLZ-75 (GenBank KT783533.1) was used as the PCR template to validate the primer. The reaction volume was 25 μl, containing 12.5 μl Green Taq mix (Vazyme, Nanjing, China), 1 μmol each primer, 2 μl extracted DNA (50 to 100 ng), and adjusted to 25 μl by ddH2O. The amplification conditions were an initial denaturation for 5 min at 94°C followed by 30 cycles for 1 min at 94°C and 30 s at 60°C. Nontemplate controls used ddH2O instead of genomic DNA.
(ii) Quantitative PCR. The qPCRs were performed in a real-time PCR system with the StepOne real-time PCR detection system and StepOne v2.0 software (Applied Biosystems, Foster City, CA). The reaction volume was 20 μl, containing 10 μl AceQ Universal SYBR green qPCR master mix (Vazyme, Nanjing, China), 4 μmol of each primer, 1 μl template, with ddH2O to make 20 μl. The amplification conditions of PLace, PPpen, PWpar, and PBcoa were 95°C for 5 min followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. The amplification conditions of PLjin were 98°C for 1 min followed by 40 cycles of 98°C for 10 s, 55°C for 30 s, and 72°C for 25 s. All samples were automatically processed for melting curve analyses to determine reaction specificity. Melting curves were obtained through slow heating from 60°C to 95°C at 5-s intervals of 0.5°C, with continuous fluorescence collection. Three replicates were performed for each experiment.
(iii) Standard curves. The recombinant plasmids were obtained by cloning targeted DNA fragments into the T-Vector pMD19 (TaKaRa, Dalian, China) following the manufacturer’s protocol. The mass of recombinant plasmids was quantified in a NanoDrop ND-1000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA) at 260 nm. The copy number (CN) of recombinant plasmid was calculated as CN = (a × 6.02 × 1014)/(b × 660), where a was the mass of recombinant plasmid (ng) and b was the length of recombinant plasmid (bp). The serial dilutions of recombinant plasmids ranging from 10.7 to 1.07 × 108 copies/μl (L. acetotolerans), 17.8 to 17.8 × 108 copies/μl (L. jinshani), 28.8 to 2.88 × 108 copies/μl (P. pentosaceus), 17 to 1.70 × 108 copies/μl (W. paramesenteroides), and 10.7 to 1.07 × 108 copies/μl (B. coagulans) were used to construct the standard quantification curves. Amplification of each dilution by qPCR was performed in quintuplicate reactions. The standard curve was built by plotting mean Ct values against the log10 of the number of copies per reaction.
(iv) Absolute quantification method for microbiota. Figure 1 shows the key steps of the absolute quantification method for microbiota. In the first step, PCR amplification of the16S rRNA gene fragments was followed by sequencing, and sequence processing of each sample. In the second step, indigenous internal standards were screened based on the microbial relative abundance and distribution frequency. Species-specific qPCR was used to detect the absolute abundance of each indigenous internal standard. The absolute abundance of each indigenous internal standard was calculated with the log10 of copies of the 16S rRNA gene. For L. acetotolerans, the copy numbers of BAQ57713.1 and 16S rRNA gene were 1 and 4 in the genome; for P. pentosaceus, the copy numbers of WP_002833728 and 16S rRNA gene were 1 and 5 in the genome; for W. paramesenteroides, the copy numbers of ATF40921.1 and 16S rRNA gene were 1 and 8 in the genome; for B. coagulans, the copy numbers of QAU28402.1 and 16S rRNA gene were 1 and 10 in the genome. In the third step, based on the multiple relationships of relative and absolute abundances of indigenous internal standards, the absolute abundances of total bacterial absolute abundance and each genus were calculated.
Standard curves of universal primers.
The universal primers were 341 F (5′-CCTACGGGAGGCAGCAG-3′) and 534 R (5′-ATTACCGCGGCTGCTGG-3′). The 16S rRNA gene sequences of Escherichia coli LBM 10017 and Enterococcus lactis LBM 10018 were cloned into T-Vector pMD19 (TaKaRa, Dalian, China) following the manufacturer’s protocol. The serial dilutions of recombinant plasmids ranging from 4.4 to 4.37 × 106 copies/μl (E. coli LBM 10017) and 2.6 to 2.57 × 106 copies/μl (E. lactis LBM 10018) were used to develop the standard curve. The reaction volume was 20 μl, containing 10 μl of AceQ Universal SYBR green qPCR master mix (Vazyme, Nanjing, China), 4 μmol of each primer, 1 μl of template, and ddH2O up to 20 μl. The amplification conditions of qPCR were 98°C for 1 min followed by 40 cycles of 98°C for 10 s and 60°C for 30 s. All samples were automatically processed for melting curve analyses to determine reaction specificity. Melting curves were obtained through slow heating from 60°C to 95°C at 5-s intervals of 0.5°C, with continuous fluorescence collection. Three replicates were performed for each experiment. The standard curve was built by plotting mean Ct values against the log10 of the number of copies per reaction.
16S rRNA amplicon sequencing and sequence processing.
The V3-V4 hypervariable region of the 16S rRNA gene was amplified by PCR (95°C for 5 min followed by 25 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 40 s, with a final extension of 72°C for 10 min) with universal primers forward 338 F and reverse 806 R (44). The PCRs were performed in a 25-μl volume, containing 2.5μl of 10× Pyrobest buffer, 2 μl of 2.5 mM dNTPs, 1 μl of each primer (10 μM), 0.4 U of Pyrobest DNA polymerase (TaKaRa, Dalian, China), 15 ng of template DNA, and ddH2O up to 25 μl. Sequencing was performed on the Illumina MiSeq for 2 × 300 bp paired-end sequencing (Illumina, San Diego, CA). The raw Miseq-generated sequence data were processed using the QIIME pipeline (45). Briefly, the sequences with ambiguous bases of n > 0, homopolymers of n > 6, primer mismatches, average quality scores of <20, and lengths (excluding the primer/barcode region) of <110 bp were removed. Forward and reverse sequences were merged with FLASH using a minimum overlap of 20 bp and zero allowed mismatches (46). Using the DADA2 pipeline, the merged sequences were denoised into their exact sequences to yield amplicon sequence variants (ASVs) and the chimeras were removed (47). A single representative sequence from each ASV was used to align to the NCBI NR database using the BLAST program. The core species that were shared in all samples were picked out. The relative abundance of one species was the sum of the relative abundance of all ASVs belonging to this species (supplemental material S2), which was in line with a previous study in which nine ASVs belonged to Veillonella parvula (48).
Metatranscriptomic sequencing and sequence processing.
Sequencing was performed on the Illumina HiSeq 4000 for 2 × 150 bp paired-end sequencing (Illumina, San Diego, CA). All the operations were performed following the manufacturer’s protocol. The clean reads were obtained by removing the reads with the N bases or adapter, low-quality reads (mean quality score Q < 20), and rRNA reads from raw reads. Clean reads were assembled using the Trinity RNA-Seq assembly algorithm to get the de novo transcript sequence (49). N50 and N75 lengths were applied to evaluate the assembly results. The open reading frame (ORF) of genes was predicted using TransGeneScan software (50). A nonredundant gene catalog was constructed using the CD-HIT software with mapping criteria of >95% identity and >90% coverage (51). Gene expression levels were estimated by FPKM using RNA-Seq by Expectation Maximization (RSEM) software (52). Taxonomic classification was performed using the BLASTP program against the NCBI NR database (E value < 10−5). The bacterial community was picked based on annotation results. Gene functional classification was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) assignments (E value < 10−5).
Metagenomic sequencing and sequence processing.
The DNA of three replicates of days 8, 15, and 40 was mixed for metagenomic sequencing, which was performed on the Illumina Hiseq 4000 for 2 × 150 bp paired-end sequencing (Illumina, San Diego, CA). For the raw data, the reads with N bases or adapter, low-quality reads (mean quality score Q < 20) were removed. The raw sequencing data of metagenomic sequencing are summarized in Table S5. The read numbers of ten core species were estimated via aligning clean reads with the NCBI NR database using Diamond software (E value < 10−5) (53).
Flavor compound determination.
Eight-gram fermented grain samples were added to 15 ml ddH2O, treated with ultrasonic waves for 30 min, and then centrifuged at 10,000 × g for 10 min. After filtering using a 0.2-nm pore size filter, the filtrate was used to analyze the concentrations of flavor compounds. Flavor compounds were detected using gas chromatography-mass spectrometry (Agilent 6890N GC system and Agilent 5975 mass selective detector, Santa Clara, CA) as previously described (54). A total of 61 flavor compounds were detected in fermentation. Among them, 20 flavor compounds were considered key flavor compounds whose odor activity value (OAV) was >1 in the product liquor (55).
Statistical analysis.
The linearity (R2) between log10 of the number of copies per reaction and Ct value was calculated in Origin 2018. The Pearson correlation coefficients (Pearson’s r) between relative and absolute abundance of microbial genera, microbial genera and flavor compounds, absolute abundances of Weissella obtained from two internal standards, and relative proportions of core species obtained from amplicon and metagenomic sequencing were calculated with SPSS Statistics 22, where Pearson’s r of >0.6 and a statistical significance of P <0.05 was considered a robust correlation (14). Visualization of correlation network between microbial genera and flavor compounds was performed using Gephi (version 0.9.1). The significance of difference of absolute abundances of Weissella obtained from two internal standards was calculated by paired-sample t tests.
Data availability.
The raw sequence data of the 16S rRNA amplicon sequencing, metatranscriptomic sequencing, and metagenomic sequencing were submitted to the DNA Data Bank of Japan (DDBJ) under the accession numbers DRA008888, DRA008889, and DRA009543, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (2018YFD0400402), the National Natural Science Foundation of China (31530055), the Jiangsu Province Science and Technology Project (BE2017705), the China Postdoctoral Science Foundation (2017M611702), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-12), and the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (number 111-2-06).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wolfe BE, Button JE, Santarelli M, Dutton RJ. 2014. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 158:422–433. doi: 10.1016/j.cell.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu ZM, Liu N, Wang LJ, Wu LH, Gong JS, Yu YJ, Li GQ, Shi JS, Xu ZH. 2016. Elucidating and regulating the acetoin production pole of microbial functional groups in multispecies acetic acid fermentation. Appl Environ Microbiol 82:5860–5868. doi: 10.1128/AEM.01331-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokulich NA, Amiranashvili L, Chitchyan K, Ghazanchyan N, Darbinyan K, Gagelidze N, Sadunishvili T, Goginyan V, Kvesitadze G, Torok T, Mills DA. 2015. Microbial biogeography of the transnational fermented milk matsoni. Food Microbiol 50:12–19. doi: 10.1016/j.fm.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 4.McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, Nuñez A, Butrym ED, Richards MP, Wang C-S, Cheng G, Zhao Z, Wang C. 2004. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci U S A 101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin G, Zhu Y, Xu Y. 2017. Mystery behind Chinese liquor fermentation. Trends Food Sci Technol 63:18–28. doi: 10.1016/j.tifs.2017.02.016. [DOI] [Google Scholar]
- 6.Liu H, Sun B. 2018. Effect of fermentation processing on the flavor of baijiu. J Agric Food Chem 66:5425–5432. doi: 10.1021/acs.jafc.8b00692. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Gifford S, Ducklow H, Schofield O, Cassar N. 2018. Towards quantitative microbiome community profiling using internal standards. Appl Environ Microbiol 85:14. doi: 10.1128/AEM.02634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandeputte D, Kathagen G, D'hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, Wang J, Tito RY, De Commer L, Darzi Y, Vermeire S, Falony G, Raes J. 2017. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Li J, Yu L, Pan H, Liu H, Liu Y, Di H, Li Y, Xu J. 2018. Simultaneous measurement of bacterial abundance and composition in response to biochar in soybean field soil using 16S rRNA gene sequencing. Land Degrad Dev 29:2172–2182. doi: 10.1002/ldr.2838. [DOI] [Google Scholar]
- 10.Caenepeel C, Vieira-Silva S, Sabino J, Machiels K, Falony G, Ferrante M, Van Assche G, Raes J, Vermeire S. 2018. Quantitative microbiome profiling changes the described dysbiotic state in inflammatory bowel disease. J Crohns Colitis 12:S548–S549. doi: 10.1093/ecco-jcc/jjx180.981. [DOI] [Google Scholar]
- 11.Vieira-Silva S, Sabino J, Valles-Colomer M, Falony G, Kathagen G, Caenepeel C, Cleynen I, van der Merwe S, Vermeire S, Raes J. 2019. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol 4:1826–1831. doi: 10.1038/s41564-019-0483-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Feng S, Wu Q, Huang H, Chen Z, Li S, Xu Y. 2019. Raw material regulates flavor formation via driving microbiota in Chinese liquor fermentation. Front Microbiol 10:1520. doi: 10.3389/fmicb.2019.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Du H, Zhang Y, Xu Y. 2017. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation. Appl Environ Microbiol 84:e02369-17. doi: 10.1128/AEM.02369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Wu Q, Nie Y, Wu J, Xu Y. 2019. Construction of synthetic microbiota for reproducible flavor metabolism in Chinese light aroma type liquor produced by solid-state fermentation. Appl Environ Microbiol 85: e03090-18. doi: 10.1128/AEM.03090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Props R, Kerckhof FM, Rubbens P, De Vrieze J, Sanabria EH, Waegeman W, Monsieurs P, Hammes F, Boon N. 2017. Absolute quantification of microbial taxon abundances. ISME J 11:584–587. doi: 10.1038/ismej.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Lou J, Wang H, Wu L, Xu J. 2018. Use of an improved high-throughput absolute abundance quantification method to characterize soil bacterial community and dynamics. Sci Total Environ 633:360–371. doi: 10.1016/j.scitotenv.2018.03.201. [DOI] [PubMed] [Google Scholar]
- 17.Calle ML. 2019. Statistical analysis of metagenomics data. Genomics Inform 17:e6. doi: 10.5808/GI.2019.17.1.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. 2017. Microbiome datasets are compositional: and this is not optional. Front Microbiol 8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stämmler F, Gläsner J, Hiergeist A, Holler E, Weber D, Oefner PJ, Gessner A, Spang R. 2016. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 4:28. doi: 10.1186/s40168-016-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tourlousse DM, Yoshiike S, Ohashi A, Matsukura S, Noda N, Sekiguchi Y. 2017. Synthetic spike-in standards for high-throughput 16S rRNA gene amplicon sequencing. Nucleic Acids Res 45:e23. doi: 10.1093/nar/gkw984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du RB, Wu Q, Xu Y. 2020. Distribution of Lactobacillus sp. in Chinese liquor fermentation system from different producing location by three-step fluorescent quantitative PCR (in Chinese). Microbiology China 47:1–12. doi: 10.13344/j.microbiol.china.190150. [DOI] [Google Scholar]
- 22.Yu Y, Li X, Zhang J, Chai LJ, Lu ZM, Xu ZH. 2020. Lactobacillus jinshani sp. nov., isolated from solid-state vinegar culture of Zhenjiang aromatic vinegar. Antonie Van Leeuwenhoek 113:43–54. doi: 10.1007/s10482-019-01316-1. [DOI] [PubMed] [Google Scholar]
- 23.Krieger CJ, Zhang P, Mueller LA, Wang A, Paley S, Arnaud M, Pick J, Rhee SY, Karp PD. 2004. MetaCyc: a multiorganism database of metabolic pathways and enzymes. Nucleic Acids Res 32:D438–D442. doi: 10.1093/nar/gkh100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandre A, Yan S. 2018. Genome watch: keeping tally in the microbiome. Nat Rev Microbiol 16:124. doi: 10.1038/nrmicro.2018.13. [DOI] [PubMed] [Google Scholar]
- 25.Li XR, Ma EB, Yan LZ, Meng H, Du XW, Zhang SW, Quan ZX. 2011. Bacterial and fungal diversity in the traditional Chinese liquor fermentation process. Int J Food Microbiol 146:31–37. doi: 10.1016/j.ijfoodmicro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Lucena-Padrós H, Ruiz-Barba JL. 2019. Microbial biogeography of Spanish-style green olive fermentations in the province of Seville, Spain. Food Microbiol 82:259–268. doi: 10.1016/j.fm.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, Jiang S, Chen J, Ma C, Huo D, Shao Y, Zhang J. 2018. Unique microbial diversity and metabolic pathway features of fermented vegetables from Hainan, China. Front Microbiol 9:399. doi: 10.3389/fmicb.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace RJ, Sasson G, Garnsworthy PC, Tapio I, Gregson E, Bani P, Huhtanen P, Bayat AR, Strozzi F, Biscarini F, Snelling TJ, Saunders N, Potterton SL, Craigon J, Minuti A, Trevisi E, Callegari ML, Cappelli FP, Cabezas-Garcia EH, Vilkki J, Pinares-Patino C, Fliegerova KO, Mrázek J, Sechovcová H, Kopečný J, Bonin A, Boyer F, Taberlet P, Kokou F, Halperin E, Williams JL, Shingfield KJ, Mizrahi I. 2019. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci Adv 5:eaav8391. doi: 10.1126/sciadv.aav8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Ning D, Zhang B, Li Y, Zhang P, Shan X, Zhang Q, Brown MR, Li Z, Van Nostrand JD, Ling F, Xiao N, Zhang Y, Vierheilig J, Wells GF, Yang Y, Deng Y, Tu Q, Wang A, Global Water Microbiome Consortium, Zhang T, He Z, Keller J, Nielsen PH, Alvarez PJJ, Criddle CS, Wagner M, Tiedje JM, He Q, Curtis TP, Stahl DA, Alvarez-Cohen L, Rittmann BE, Wen X, Zhou J. 2019. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat Microbiol 4:2579–2579. doi: 10.1038/s41564-019-0617-0. [DOI] [PubMed] [Google Scholar]
- 30.Lou J, Yang L, Wang H, Wu L, Xu J. 2018. Assessing soil bacterial community and dynamics by integrated high-throughput absolute abundance quantification. PeerJ 6:e4514. doi: 10.7717/peerj.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polz MF, Cavanaugh CM. 1998. Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64:3724–3730. doi: 10.1128/AEM.64.10.3724-3730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wear EK, Wilbanks EG, Nelson CE, Carlson CA. 2018. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ Microbiol 20:2709–2726. doi: 10.1111/1462-2920.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piwosz K, Shabarova T, Tomasch J, Šimek K, Kopejtka K, Kahl S, Pieper DH, Koblížek M. 2018. Determining lineage-specific bacterial growth curves with a novel approach based on amplicon reads normalization using internal standard (ARNIS). ISME J 12:2640–2654. doi: 10.1038/s41396-018-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Z, Du H, Zhang Y, Xu Y. 2017. Unraveling core functional microbiota in traditional solid-state fermentation by high-throughput amplicons and metatranscriptomics sequencing. Front Microbiol 8:1294. doi: 10.3389/fmicb.2017.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, Harris HMB, McCann A, Guo C, Argimón S, Zhang W, Yang X, Jeffery IB, Cooney JC, Kagawa TF, Liu W, Song Y, Salvetti E, Wrobel A, Rasinkangas P, Parkhill J, Rea MC, O'Sullivan O, Ritari J, Douillard FP, Paul Ross R, Yang R, Briner AE, Felis GE, de Vos WM, Barrangou R, Klaenhammer TR, Caufield PW, Cui Y, Zhang H, O'Toole PW. 2015. Expanding the biotechnology potential of Lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun 6:8322. doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvetti E, Torriani S, Felis GE. 2012. The genus Lactobacillus: a taxonomic update. Probiotics Antimicrob Proteins 4:217–226. doi: 10.1007/s12602-012-9117-8. [DOI] [PubMed] [Google Scholar]
- 37.Wu LH, Lu ZM, Zhang XJ, Wang ZM, Yu YJ, Shi JS, Xu ZH. 2017. Metagenomics reveals flavour metabolic network of cereal vinegar microbiota. Food Microbiol 62:23–31. doi: 10.1016/j.fm.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Kaseleht K, Paalme T, Mihhalevski A, Sarand I. 2011. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int J Food Sci Technol 46:1940–1946. doi: 10.1111/j.1365-2621.2011.02705.x. [DOI] [Google Scholar]
- 39.Jung JY, Lee SH, Jin HM, Hahn Y, Madsen EL, Jeon CO. 2013. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int J Food Microbiol 163:171–179. doi: 10.1016/j.ijfoodmicro.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 40.You FM, Huo N, Gu YQ, Luo M-C, Ma Y, Hane D, Lazo GR, Dvorak J, Anderson OD. 2008. BatchPrimer3: a high throughput web application for PCR and sequencing primer design. BMC Bioinformatics 9:253. doi: 10.1186/1471-2105-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson DM, Muck RE, Shinners KJ, Weimer PJ. 2006. Use of real time PCR to determine population profiles of individual species of lactic acid bacteria in alfalfa silage and stored corn stover. Appl Microbiol Biotechnol 71:329–338. doi: 10.1007/s00253-005-0170-z. [DOI] [PubMed] [Google Scholar]
- 42.Yan T, Zhu J, Jiang T, Chen K, Fang S. 2018. Isolation and optimization on spore-forming conditions of Bacillus coagulans. Microbiology China 45:238–249. [Google Scholar]
- 43.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soergel DAW, Dey N, Knight R, Brenner SE. 2012. Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J 6:1440–1444. doi: 10.1038/ismej.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Nanayakkara S, Gao JL, Nguyen KA, Adler CJ. 2019. Storage media and not extraction method has the biggest impact on recovery of bacteria from the oral microbiome. Sci Rep 9:10. doi: 10.1038/s41598-019-51448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, MacManes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, Leduc RD, Friedman N, Regev A. 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ismail WM, Ye Y, Tang H. 2014. Gene finding in metatranscriptomic sequences. Bioinformatics 15:8. doi: 10.1186/1471-2105-15-S9-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 52.Li B, Dewey CN. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 54.Wang P, Wu Q, Jiang X, Wang Z, Tang J, Xu Y. 2017. Bacillus licheniformis affects the microbial community and metabolic profile in the spontaneous fermentation of Daqu starter for Chinese liquor making. Int J Food Microbiol 250:59–67. doi: 10.1016/j.ijfoodmicro.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Sun B, Zhao M, Zheng F, Huang M, Sun J, Sun X, Li H. 2016. Characterization of the key odorants in Chinese Zhima aroma-type Baijiu by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J Agric Food Chem 64:5367–5374. doi: 10.1021/acs.jafc.6b01390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data of the 16S rRNA amplicon sequencing, metatranscriptomic sequencing, and metagenomic sequencing were submitted to the DNA Data Bank of Japan (DDBJ) under the accession numbers DRA008888, DRA008889, and DRA009543, respectively.