Humans have mastered high-salinity fermentation techniques for bean-based fermented product preparation over thousands of years. High salinity was used to select the functional microbiota and conducted food fermentation production with unique flavor. Although a high-salinity environment is beneficial for suppressing harmful microbes in the open fermentation environment, the fermentation efficiency of functional microbes is partially inhibited. Therefore, application of defined starter cultures for reduced-salt fermentation in a sterile environment is an alternative approach to improve the fermentation efficiency of bean-based fermented foods and guide the transformation of traditional industry. However, the assembly and function of self-organized microbiota in an open fermentation environment are still unclear. This study provides a comprehensive understanding of microbial function and the mechanism of community succession in a high-salinity environment during the fermentation of broad bean paste so as to reconstruct the microbial community and realize efficient fermentation of broad bean paste in a sterile environment.
KEYWORDS: Chinese broad bean paste, microbial community structure, community driving force, microbial functions, reconstruction of synthetic microbial community
ABSTRACT
Humans have used high salinity for the production of bean-based fermented foods over thousands of years. Although high salinity can inhibit the growth of harmful microbes and select functional microbiota in an open environment, it also affects fermentation efficiency of bean-based fermented foods and has a negative impact on people’s health. Therefore, it is imperative to develop novel defined starter cultures for reduced-salt fermentation in a sterile environment. Here, we explored the microbial assembly and function in the fermentation of traditional Chinese broad bean paste with 12% salinity. The results revealed that the salinity and microbial interactions together drove the dynamic of community and pointed out that five dominant genera (Staphylococcus, Bacillus, Weissella, Aspergillus, and Zygosaccharomyces) may play different key roles in different fermentation stages. Then, core species were isolated from broad bean paste, and their salinity tolerance, interactions, and metabolic characteristics were evaluated. The results provided an opportunity to validate in situ predictions through in vitro dissection of microbial assembly and function. Last, we reconstructed the synthetic microbial community with five strains (Aspergillus oryzae, Bacillus subtilis, Staphylococcus gallinarum, Weissella confusa, and Zygosaccharomyces rouxii) under different salinities and realized efficient fermentation of broad bean paste for 6 weeks in a sterile environment with 6% salinity. In general, this work provided a bottom-up approach for the development of a simplified microbial community model with desired functions to improve the fermentation efficiency of bean-based fermented foods by deconstructing and reconstructing the microbial structure and function.
IMPORTANCE Humans have mastered high-salinity fermentation techniques for bean-based fermented product preparation over thousands of years. High salinity was used to select the functional microbiota and conducted food fermentation production with unique flavor. Although a high-salinity environment is beneficial for suppressing harmful microbes in the open fermentation environment, the fermentation efficiency of functional microbes is partially inhibited. Therefore, application of defined starter cultures for reduced-salt fermentation in a sterile environment is an alternative approach to improve the fermentation efficiency of bean-based fermented foods and guide the transformation of traditional industry. However, the assembly and function of self-organized microbiota in an open fermentation environment are still unclear. This study provides a comprehensive understanding of microbial function and the mechanism of community succession in a high-salinity environment during the fermentation of broad bean paste so as to reconstruct the microbial community and realize efficient fermentation of broad bean paste in a sterile environment.
INTRODUCTION
Traditional bean-based fermented foods have a long history of thousands of years. Through the summarization of consistent practice experiences, the salinity of 12% to 15% was optimized so as to select the salt-tolerant functional microbial community and inhibit the growth of miscellaneous microbes with nonsterile raw materials in an open fermentation environment. Bean-based fermented foods such as broad bean paste (BBP), soybean paste (doenjang), soy sauce, and natto are very popular in Asia. Chinese BBP is a traditional aliment, which is usually made from broad beans and wheat flour via fermentation by various groups of microorganisms. In general, the production of traditional BBP is characterized by an open process of multispecies solid-state fermentation and mainly includes two steps. First, for making koji, broad bean is pretreated by steaming, followed by inoculating with Aspergillus oryzae and fermenting for a couple of days. In the second step, koji is fermented again after mixing with salt water in a ceramic large tank (the mixture termed Pei in Chinese), and the obtained Pei is exposed to the sun for 2 to 6 months with periodic manual pouring during fermentation.
Salt is a major ingredient during the BBP fermentation, which plays a key role in selecting salt-tolerant functional microbiota and inhibiting the growth of spoilage and pathogenic microbes in an open fermentation environment (1). However, high salinity also inhibits the fermentation efficiency of functional microbes, resulting in longer fermentation cycles (2). In addition, recent studies found that high-salt diets are related to increased risk of hypertension, cardiovascular mortality (3), and obesity (4), and so it is beneficial to reduce the salt content of bean-based fermented foods. However, spontaneous fermentation with low salinity often leads to growth of harmful microorganisms, resulting in low-quality and off-flavor products (5). Therefore, it is imperative to develop novel defined starter cultures for reduced-salt fermentation in a sterile environment. Previous studies have provided a preliminary view of the microbial community structure and potential function existing in bean-based fermented products based on pure culture (6), PCR-denaturing gradient gel electrophoresis (DGGE) (7, 8), and the rapid and accurate next-generation sequencing technology (9, 10). However, the mechanisms of microbial community assembly and function are still unclear due to the gap between the in situ analyses of patterns and mechanisms that can explain these patterns (11). In recent years, advances in multi-omics (e.g., metagenomics, metatranscriptomics, metabolomics, and culturomics) have provided opportunities to investigate the phenotypes and functions of microbial communities (12) and bridge the gap between observations and mechanisms by developing a microbial community model with desired functions (13). For example, Dutton et al. developed the cheese rind microbial communities as an experimentally tractable system to determine the mechanisms affecting the assembly and function of microbial communities (14). Similarly, the fermentation of BBP involves a relatively simple microbial community due to its high salinity, which also represents a potential model ecosystem. Species quantification of amplicon sequencing is based on relative abundance, which neglects the absolute number of the community, and cannot accurately describe the interaction between microorganisms or the interaction between microorganisms and physicochemical metabolites (15, 16). Here, we carried out both relative and absolute quantitative analysis of the dominant genera by amplicon sequencing and quantitative real-time PCR so that the changes of taxon abundance could be depicted closer to the real situation.
In this study, we were interested in screening the functional microorganisms of BBP during fermentation and obtaining the synthetic microbial community so as to realize the fermentation of bean-based fermented foods with low salinity in a sterile environment. First, relative and absolute quantitative analyses revealed the structure and succession of the microbial community during BBP fermentation. Then, a correlation analysis explored the main factors driving community variation and predicted the potential function of microorganisms. Second, based on the functional analysis of the microbial community, we identified and isolated the key microbial strains with fermentation function and evaluated their salt tolerance, bacteriostatic, and metabolic characteristics. Finally, based on an interaction analysis, we reconstructed the microbial community under a different salinity and realized efficient fermentation of BBP in a sterile environment with low salinity. Collectively, taking BBP as a model system, the structure and function were deconstructed and reconstructed in situ and in vitro, which is better to understand the underlying mechanisms of microbial assembly and functions in an open fermentation environment. In addition, the reconstruction of microbial community will help to develop novel defined starter cultures to improve the fermentation efficiency of bean-based fermented foods and guide the transformation of traditional bean-based fermented industry.
RESULTS
Temporal changes of physicochemical parameters and flavor metabolites.
In this study, moisture, salinity, pH, titratable acidity, amino acid nitrogen, reducing sugar content, protease activity, and glucoamylase activity were analyzed (Fig. 1). The moisture of BBP changed greatly in the first 3 days due to the sharp water absorption of the koji and then remained steady around 55% upon further fermentation, which was mainly related to the unique repeated sunning and night dew process during the natural fermentation. The salinity increased from 0% to 11.8% (wt/wt) in the first 2 days due to the permeation of sodium chloride into the koji and remained stable around 12% to 13% (wt/wt). Titratable acidity increased from 3.08% to 5.68% (wt/wt), resulting in a pH value decrease from 6.4 to 5.5. With the development of fermentation, the content of amino acid nitrogen increased rapidly and achieved the maximal level of 1.96% (wt/wt) on day 41, while the protease activity decreased sharply when koji was mixed with sodium chloride at a high concentration (approximately 20% to 22% [wt/vol] NaCl). The contents of amino acids are usually used as a vital indicator to determine the quality level of bean-based products, and the activity of protease directly affects their formation (17). In addition, the glucoamylase activity increased rapidly at the beginning of fermentation. At the same time, the content of reducing sugar ramped from 14.0% to 23.1% (wt/wt) in the first 2 days of fermentation, which was due to the decomposition of starch by glucoamylase secreted by Aspergillus, resulting in a formation rate of reducing sugar far greater than the consumption rate. Later, the content of reducing sugar decreased slightly because of the decrease of glucoamylase activity, utilization of reducing sugar by microorganisms, and the Maillard reaction (18).
FIG 1.
Temporal changes of physicochemical parameters and flavor metabolites during the BBP fermentation. (A) Moisture content. (B) Salinity. (C) Titratable acidity and pH. (D) Amino acid nitrogen content. (E) Reducing sugar content. (F) Protease activity and glucoamylase activity. (G) Free amino acids. (H) Nonvolatile organic acids. (I) Volatile flavor compounds. The peak area of each volatile flavor compound was normalized using Z-scores. The color intensity is proportional to the relative abundance of volatile compounds. K represents koji.
The flavor metabolites during the BBP fermentation were also detected, including 17 amino acids, 6 organic acids, and 107 volatile compounds. Amino acids can enhance the taste (e.g., umami) of BBP and be further metabolized to volatile flavor metabolites (19). As shown in Fig. 1G, amino acids increased to the maximal level in the middle stage and then decreased in the later stage of fermentation. Glutamic acid was the most abundant amino acid of BBP, accounting for 16.8% of the total content. The release of glutamic acid produced by glutaminase is essential for the umami taste of bean-based products (20). Organic acids can balance the taste of BBP by adjusting the acidity (21) and participate in the synthesis of aromatic esters as precursors (22). The levels of organic acids were sharply increased in the first week, followed by a brief decline, and then continued to rise (Fig. 1H). Acetic acid and lactic acid were the predominant organic acids, representing more than 90% of the total content, which have great influences on the flavor and shelf life of fermented foods (23, 24). The volatile compounds during the BBP fermentation were detected by headspace solid-phase microextraction-gas chromatography mass spectrometry (HS-SPME/GC-MS). A total of 107 volatile compounds were identified, mainly including 19 alcohols, 41 esters, 12 aldehydes, 12 ketones, 6 phenols, 2 acids, and 15 other low-content compounds (Fig. 1I). Among them, alcohols and aromatic esters were regarded as the most abundant and important groups determining the flavor characteristics of BBP (25). Alcohols remarkably influence the smell and taste of paste while they also participate in the synthesis of desirable esters in BBP (22). Aromatic esters with fruity and flowery aromas are conducive to improve the aromatic complexity of BBP (26). The heatmap of the volatile compounds indicated that most of the volatile compounds showed a rising trend during fermentation, and we found the mid-late stage of fermentation was an important period for the generation and accumulation of flavor substances. Furthermore, principal-component analysis (PCA) revealed significant variances in the compositions of volatile substances among different fermentation stages, and the compositions of volatile flavor substances tended to be stable at the final stage of fermentation (see Fig. S2 in the supplemental material).
Structure and succession of microbial community.
In this study, the structure and succession of bacterial and fungal communities in BBP were analyzed by amplicon sequencing. According to the results, 231 bacterial operational taxonomic units (OTUs) and 70 fungal OTUs were obtained from all samples, which were distributed in 43 bacterial and 25 fungal genera, respectively. As shown in Fig. S3, the diversity of the bacterial community peaked on day 2 due to the introduction of environmental microorganisms and then declined, possibly due to the intolerance to environmental pressure, while the diversity of fungal communities remained relatively stable throughout the fermentation. According to the principal-component analysis (PCA), both bacterial and fungal communities changed significantly in the early stage of fermentation and then stabilized in the mid-late stage. By clustering, the fermentation process was divided into three stages: koji stage (K), initial stage (days 0 to 5), and mid-late stage (days 6 to 55). Through statistical analysis of all samples, 5 bacterial and 2 fungal genera were found at greater than 1% average abundance, which were defined as dominant genera (14), including Staphylococcus (79.8%), Bacillus (7.5%), Weissella (5.9%), unidentified Bacillales (3.0%), unidentified Bacilli (1.7%), Aspergillus (97.0%), and Zygosaccharomyces (1.3%) (see Table S1). These dominant genera were widely distributed throughout the fermentation, and their relative abundances were variable at different fermentation stages.
However, the species quantification described above based on amplicon sequencing was relative and cannot represent the actual abundance of microbes. Only when these compositions are combined with absolute quantification can the changes of taxon abundance be described more accurately (27). Therefore, we conducted an absolute quantitative analysis of dominant genera through quantitative real-time PCR and realized the transformation from relative quantitation to absolute quantitation. As shown in Fig. 2A, bacterial biomass and composition changed dramatically in the initial stage of fermentation and stabilized in the mid-late stage. The biomass of Staphylococcus declined in the first 2 weeks of fermentation and then slowly recovered, while the biomasses of Bacillus and Weissella decreased during fermentation. In addition, fungal biomasses continued to decline during the fermentation, among which Aspergillus disappeared rapidly, while Zygosaccharomyces increased slowly during the initial stage, reached the peak in the middle stage, and then declined (Fig. 2B).
FIG 2.
Succession and driving force of microbial community during the BBP fermentation. (A) Absolute quantification of bacterial biomass. (B) Absolute quantification of fungal biomass. Data are presented as mean values from duplicates. (C) RDA based on relative abundance of dominant genera. (D) RDA based on absolute quantification of dominant genera. Red arrows represent different environmental variables. Black arrows represent dominant genera. Green, blue, and orange symbols represent the koji stage, initial stage, and mid-late stage, respectively. (E) Correlation network between different genera. Statistically significant (P < 0.05) Spearman’s correlation coefficient (|ρ| > 0.6) indicates the robust correlation. The size of nodes indicates the degree of connections. Blue and orange nodes indicate bacteria and fungi, respectively. Edge thickness represents the proportion to the value of Spearman’s correlation. Green and red edges indicate negative and positive interactions between nodes. (F) Prediction of microbial interactions between dominant genera. Pairwise correlations were calculated based on relative abundance (lower triangle) and absolute quantification (upper triangle). The correlation coefficient is represented by the color and size of the circles. Dark blue indicates positive correlation and dark red indicates negative correlation. P values were calculated using Spearman’s rank correlation test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Abiotic and biotic drivers of microbial community.
During fermentation, moisture contents, titratable acidity, pH, salinity, and ethanol contents were significantly different throughout the fermentation (Fig. 1). Some of these environmental variables correlate with community dynamics. As shown in Fig. S4, the changing trend of OTUs was related to their adaptability to environmental conditions. For example, Staphylococcus (OTU432, OTU3, OTU475, OTU458, OTU100, OTU415, and OTU474), Aspergillus (OTU125), and Zygosaccharomyces (OTU1 and OTU153) were positively correlated with salinity, while Bacillus (OTU2, OTU15, OTU79, OTU78, OTU408, and OTU12), unidentified Bacillales (OTU435 and OTU391), Weissella (OTU1 and OTU44), and Aspergillus (OTU140) were negatively correlated with salinity. Subsequently, partial redundancy analysis (RDA) was used to reveal the influences of environmental factors on the dominant genera (Fig. 2C and D). Concentrations of moisture, NaCl, titratable acid, and ethanol were positively correlated with Staphylococcus and Zygosaccharomyces while negatively correlated with Aspergillus, Bacillus, and Weissella, indicating that these four environmental factors significantly affected the abundance changes of dominant genera. RDA indicated that Staphylococcus and Zygosaccharomyces may tolerate osmotic stress, which is consistent with previous studies (28, 29). Among them, salinity had the greatest influence on the dominant genera, and the explained variation for community variation was 28.3% (based on relative abundance) or 79.3% (based on absolute quantification), which could be considered the main factor driving community succession (see Table S2). Furthermore, Spearman’s correlation analysis between community succession and environmental variables by the Mantel test demonstrated that salinity was significantly correlated with bacterial community succession (|ρ| > 0.60, P < 0.001) and that ethanol had a significant correlation with fungal microbial community succession (|ρ| = 0.60, P < 0.001) (Table 1). It can be inferred that abiotic factors had a strong influence on the succession of microbial communities during the BBP fermentation, i.e., the growth of bacteria was mainly inhibited by salinity, while the growth of fungi was mainly limited by ethanol.
TABLE 1.
Correlations between microbial community succession and environmental variables during the fermentation process analyzed by Mantel test
| Variable | Spearman’s value (|ρ|)a
|
|||
|---|---|---|---|---|
| Bacteria |
Fungi |
|||
| Relative | Absolute | Relative | Absolute | |
| Moisture | 0.53*** | 0.52*** | 0.08*** | 0.42*** |
| Salinity | 0.62*** | 0.69*** | 0.09*** | 0.49*** |
| Alcohol | 0.29*** | 0.26*** | 0.23*** | 0.60*** |
| Titratable acid | 0.29*** | 0.28*** | 0.16*** | 0.47*** |
Correlations were calculated based on relative abundance and absolute quantification. ***, P < 0.001. Significant correlations are in bold.
Biotic factors can underpin the distribution and efficacy of the microbial community. Recent research has demonstrated that cooccurring taxa exert considerable influence on microbial structure and function regardless of their abundance across space and time (30). Network analysis facilitates the exploration of many interconnected correlations simultaneously and has become a useful tool for inferring cooccurring taxa from complex biological systems (31). Based on the microbial network analysis, the Spearman’s correlation coefficients of 68 genera were calculated, and correlations among different genera were complicated (Fig. 2E). A total of 39 nodes (23 nodes for bacteria and 16 nodes for fungi) and 121 pairs of significant and robust edges (25 pairs of positive correlations and 96 pairs of negative correlations) were obtained (|ρ| > 0.6, P < 0.05). In this study, the nodes with degree of >10 were defined as cooccurring genera (31). As shown in Table S3, a total of 6 bacterial genera, including Weissella, Staphylococcus, Klebsiella, unidentified Enterobacteriaceae, Erwinia, and Bacillus, and 6 fungal genera, including Lichtheimia, Agaricostilbum, Termitomyces, Cladosporium, Zygosaccharomyces, and Aspergillus, were defined as the cooccurring genera. These cooccurring genera also underlined the importance of numerically inconspicuous taxa for maintaining the structural and functional stability of the microbial community during the BBP fermentation (32). For example, Agaricostilbum and Cladosporium may play pivotal roles in complex fermentation systems, although they accounted for only 0.03% to 0.04% of the total abundance of fungi (Table S3). In addition, we compared the cooccurrence patterns across dominant genera based on different quantification methods of microbial abundance and found some discrepancies (Fig. 2F). For example, analysis using relative abundance showed a positive correlation between Staphylococcus and Zygosaccharomyces but a negative correlation under the absolute quantitative condition. In the microbial world, the prediction of microbial interaction merely using correlation analysis may be biased by habitat filtering and show positive correlations between noninteracting microbial members (30). The above discussion found that the environmental factors and microbial interaction had a potential impact on the microbial community, but there may be other factors due to the complexity of the environment.
Functional potential of microbial community.
In addition to exploring how the microbiota varies with fermentation, we also investigated the functional potential of microbial community. Based on the correlation analysis between relative abundance of dominant genera and physicochemical metabolites, we found that Bacillus, Weissella, and Aspergillus were mainly positively correlated with enzyme activity, while Staphylococcus and Zygosaccharomyces were positively correlated with titratable acid, amino acid nitrogen, free amino acids, volatile flavor substances, and organic acids (Fig. 3A). The network correlation analysis indicated that Staphylococcus and Zygosaccharomyces were significantly correlated with 64 and 56 kinds of flavor substances, respectively (Fig. 3B and Table S4). However, there was no significantly positive correlation between Staphylococcus and physicochemical metabolites according to the correlation analysis between absolute quantification of dominant genera and physicochemical metabolites (Fig. 3A). Subsequently, metagenomics revealed that microbial carbohydrate metabolism and amino acid metabolism pathways were significantly enriched during the BBP fermentation (see Fig. S5). Furthermore, species and functional contribution analysis revealed that Staphylococcus, Bacillus, Weissella, and Aspergillus were the main contributors of metabolic functions, indicating that dominant genera indeed played key roles during the BBP fermentation (Fig. 3C). In conclusion, it can be preliminarily concluded that Aspergillus, Bacillus, and Weissella mainly degraded macromolecule substances in the early stage of fermentation, while salt-tolerant Staphylococcus and Zygosaccharomyces played pivotal roles in the formation of flavor substances in the mid-late stages of fermentation.
FIG 3.
Functional potential of microbial community. (A) Spearman correlations between dominant genera and physicochemical variables during fermentation process. The correlation coefficients are represented by the color and size of the circles. Dark blue indicates positive correlation and dark red indicates negative correlation. P values were calculated using Spearman’s rank correlation test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Correlation network between microbial genera and volatile flavor compounds analyzed by Spearman’s correlation rank test (P < 0.05 and |ρ| > 0.6). The size of nodes indicates the degree of connections. Blue, orange, and yellow nodes indicate bacteria, fungi, and volatile flavor compounds, respectively. Edge thickness represents the proportion to the value of Spearman’s correlation. (C) Relative contribution of different taxa at the genus level to different function categories. Data are presented as mean values from duplicates.
Isolation and characterization of core microbes.
Statistical methods provide an opportunity to bridge the gap between the phenotype and the genotype in a fermentation ecosystem and point to previously unknown interactions. However, correlation analysis does not mean causation, and further research should be focused on verifying the functions and characteristics of core microbiota by in vitro simulation of fermentation. Thus, we isolated the core microbes from the BBP and obtained the representative species (A. oryzae, Bacillus subtilis, Staphylococcus gallinarum, Weissella confusa, and Zygosaccharomyces rouxii) with the highest relative abundance in each corresponding genus (see Table S5). Due to the species diversity of microbial genera and the limitations of microbial culture techniques, these five species were used to represent the core taxa (14, 31).
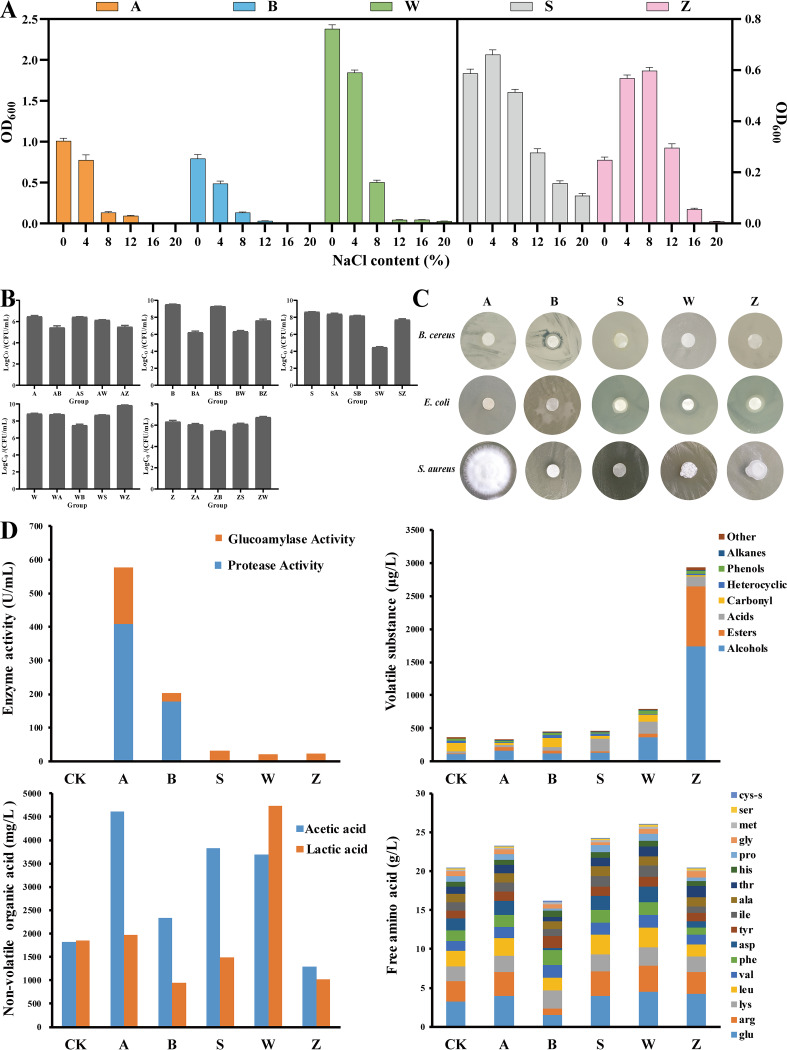
The above-described analysis showed that salinity had a significant influence on microbial community. To verify this result, we analyzed the growth status of these five strains under different salinity conditions to evaluate their salt tolerance (Fig. 4A). All of the strains sustained growth on the medium containing 8% NaCl; Z. rouxii and S. gallinarum also showed growth under 12% and 20% NaCl conditions, respectively. The salt tolerance of these strains can explain their succession during the fermentation. Among them, A. oryzae, B. subtilis, and W. confusa with low salt tolerance (8% NaCl) stopped growing during the mid-late stage of Pei fermentation, which generally occurs at an NaCl concentration of >12% (wt/wt), but they could actively be involved in koji ripening and the initial stage of Pei fermentation. Z. rouxii and S. gallinarum exhibited halotolerant growth, and the salt tolerance was high enough to involve in Pei ripening. The salt tolerance of S. gallinarum (up to 20% NaCl) was greater than that of other strains, which reinforced its dominant position during a long fermentation period. The salt tolerance of representative species observed by in vitro simulated fermentation mirrored the salt tolerance of dominant genera predicted by correlation analysis.
FIG 4.
Characteristics and functions of dominant species. (A) Growth status of dominant species in liquid media with different salinities. (B) Populations of single and double combinations of five dominant species at the end of different mixed-culture fermentations. The initial cell density of each species was adjusted to 1 × 106 CFU/ml. (C) Bacteriostatic effect of five dominant species on harmful bacteria. (D) Functions of dominant species in liquid simulation fermentation. The capital letters represent abbreviations for dominant species. A, A. oryzae; B, B. subtilis; S, S. gallinarum; W, W. confusa; Z, Z. rouxii; CK, control.
Microbial interactions can influence the structures and metabolic activities of the whole microbial community by affecting microbial growth. Therefore, we carried out in vitro mono- and biculture fermentations to verify the actual interactions between different species. The growth of each species is shown in Fig. 4B. A. oryzae and B. subtilis inhibited the growth of each other, and similar competition between B. subtilis and W. confusa also existed. S. gallinarum was significantly influenced by W. confusa, and its population decreased by nearly 50% when cocultured with W. confusa. In addition, B. subtilis has a certain inhibitory effect on most microbes, because it can secrete a variety of antibacterial substances (33). Interestingly, there was a significant interaction between W. confusa and Z. rouxii. Such lactic acid bacterium (LAB) and yeast interactions are universal in fermented foods (34, 35). The comparisons of the results of microbial interactions from in vitro validation to the predictions based on in situ correlations revealed some differences. Among them, the cooccurrence observed only from in situ analysis could be explained by the environmental preference (niche overlap) of individual species rather than direct interactions (36, 37). Otherwise, the presence of other species would also modulate microbial interactions, such as numerically inconspicuous “cooccurring populations” that may also lead to inconsistencies between in situ predictions and in vitro assays (30). Notably, albeit a minority, foodborne pathogens (Bacillus cereus, Staphylococcus aureus, and Escherichia coli) from the environment were present in the paste. As shown in Fig. 4C, we studied the bacteriostatic effects of these five strains on common harmful bacteria during the BBP fermentation. It was found that B. subtilis can inhibit the growth of B. cereus, S. gallinarum can inhibit S. aureus, and W. confusa can inhibit E. coli and S. aureus. The inhibition effect of core microbes on pathogens can also be used to explain the “systematic self-domestication” of the community.
Subsequently, we verified the metabolic characteristics of core species by in vitro simulation fermentation. As shown in Fig. 4D, A. oryzae can secrete protease and glucoamylase with high enzyme activity. B. subtilis can also secrete certain enzymes, and it inhibited the growth of A. oryzae. In addition, ester and alcohol compounds were significantly increased in the medium inoculated with Z. rouxii. We also found that A. oryzae and S. gallinarum can produce acetic acid, while W. confusa can produce not only acetic acid but also large amounts of lactic acid. This indicated that W. confusa, A. oryzae, and S. gallinarum had high organic acid production capacity, and these organic acids further led to the decrease of pH during the BBP fermentation. W. confusa, A. oryzae, and S. gallinarum also displayed high amino acid production capacity or protein degradation capacity. In general, culturomics provided an opportunity to link in situ predictions to in vitro assays of microbial functions, and partial results of microbial functions obtained from in vitro assays were consistent with the predictions based on correlations from in situ patterns. For example, A. oryzae and B. subtilis can secrete protease and glucoamylase with high enzyme activity, which contributed to the degradation of macromolecules. Z. rouxii was responsible for the formation of most volatile flavor components, which may directly lead to the unique flavor of BBP. However, we observed some apparent discrepancies between in vitro assays and in situ patterns, which may be due to the lag effect of microbial metabolic activity or the synergistic effect of multiple strains that led to the inconsistency between microbial succession and physicochemical metabolism dynamics.
Reconstruction of communities in vitro.
Based on the microbial interactions and microbial functions, we reconstructed the microbial community with desired functions and studied the effects of divergent salt concentrations on fermentation microbiota and flavor metabolites. We inoculated five core species (A. oryzae, B. subtilis, S. gallinarum, W. confusa, and Z. rouxii) with initial ratios according to the in situ fermentation into the mixed Pei with different salinities (6%, 9%, and 12%) and determined the profiles of microbial community and physicochemical properties over time. Microbial community succession in in vitro assays exhibited a similar pattern to that of the in situ fermentation process. Like the microbial community succession observed from in situ patterns, continuous declines of the biomasses of A. oryzae, B. subtilis, and W. confusa were observed in in vitro assays. In the L (low-salinity) and M (medium-salinity) groups, S. gallinarum showed a gradual growth trend during the fermentation, while in the H (high-salinity) and T (traditional) groups, the growth of S. gallinarum had an adaptation period in the early stage of fermentation and then showed an upward trend (Fig. 5). Partial death of S. gallinarum was possibly attributed to the instantaneous stress of high salinity, but then the salt tolerance of S. gallinarum was induced to make it grow gradually. In addition, in the L and M groups, the abundance of Z. rouxii did not increase significantly during the whole fermentation process, while in the H and T groups, Z. rouxii had a short period of growth in the middle stage, matching patterns observed in in situ fermentation in which this microbe was positively correlated with salinity. Furthermore, analysis of similarity (ANOSIM) was used to compare the differences in the succession of the synthetic microbial community under different salinity conditions. As shown in Fig. 5B, it was found that the differences among three treatment groups were larger than the differences within the groups (R = 0.306, P = 0.001), suggesting significant differences in the succession of the microbial community under different salinity conditions. This also indicated that the community dynamics were driven by salinity, and species with strong salt tolerance gradually replaced those with poor salt tolerance. Among them, the community successions of the L and M groups were similar (R = −0.022, P = 0.603), but there were significant differences between the L and H groups (R = 0.50, P = 0.001) (see Table S6). In addition, the microbial dynamics of in vitro fermentation under high salinity was similar to that of the in situ BBP fermentation process (R = 0.11, P = 0.041) (Table S6). Finally, we compared the fermentation performance of synthetic microbial community under different salinity fermentation conditions. As can be seen from Fig. 5C, lower salinity led to higher production rate and contents of titratable acid and amino acid nitrogen and higher contents of flavor substances, indicating that lower salinity can effectively accelerate the fermentation efficiency. Additionally, the flavor profile of BBP fermented with a synthetic microbial community had similar compositions with those of the traditional fermented BBP (Fig. 6), in which alcohols and esters accounted for more than 80% of the total flavor compounds. The results indicated that the flavor profile could be reproduced by synthetic microbial community. Therefore, it is feasible to use the synthetic microbial community for reduced-salt fermentation in a sterile environment. Based on the response of microbiota and flavor metabolites to salt concentration, it has certain guiding significance for the industrialization of bean-based fermented foods.
FIG 5.
Community reconstruction with different salinities. (A) The dynamics of microbial biomass in traditional and synthetic communities. The biomass was normalized per species using the Z-score. A, A. oryzae; B, B. subtilis; S, S. gallinarum; W, W. confusa; Z, Z. rouxii; L, low salinity (6%); M, middle salinity (9%); H, high salinity (12%); T, traditional microbial community; 0 to 41, fermentation days. (B) ANOSIM test for differences in synthetic microbial communities with different salinities. (C) The dynamics of fermentation characteristics and flavor metabolism in a synthetic community with different salinities. L, low salinity (6%); M, middle salinity (9%); H, high salinity (12%); A, noninoculated Pei.
FIG 6.
Proportions of flavor compounds in the traditional and synthetic microbial communities.
DISCUSSION
The dominant species can be defined as the widely distributed species with a higher relative abundance than others, which may play essential roles in biological processes (14). Amplicon sequencing analysis revealed 7 widely distributed genera (Staphylococcus, Bacillus, Weissella, unidentified Bacillales, unidentified Bacilli, Aspergillus, and Zygosaccharomyces) as dominant community members (see Table S3 in the supplemental material). Furthermore, quantitative PCR (qPCR) was used to quantify the actual abundances of the 5 dominant genera (Staphylococcus, Bacillus, Weissella, Aspergillus, and Zygosaccharomyces), and it was found that microbial biomass declined in the initial stage of fermentation and stabilized in the mid-late stage (Fig. 2). The absolute abundance of Staphylococcus declined in the first 2 weeks of fermentation and then gradually recovered, while Aspergillus, Bacillus, and Weissella decreased rapidly during fermentation. Zygosaccharomyces increased slowly during the fermentation, reached the peak in the middle stage of fermentation, and then declined. Although there are differences in microbial community structure among samples from different regions or bean-based fermentation foods (7, 8, 38), the dominant genera were generally the same and had universality, mainly including Staphylococcus, Bacillus, Weissella, Aspergillus, and Zygosaccharomyces. Moreover, Staphylococcus was also identified as the predominant genus of other protein-based fermentation foods such as cheese (14).
The dynamics of microbiota during the BBP fermentation can be explained by the “systematic self-domestication” or “systematic robustness principle” (39). There are several factors that can explain the systematic self-domestication of the microbial community. In the production process of BBP, salinity and environmental microorganisms can be introduced into the fermentation process by mixing koji with high-concentration brine and periodic manual pouring, which might cause a disturbance to the microbial community and make it reach a steady state rapidly (Fig. S3A and Fig. 2). Particularly, salinity had a selective filtering effect on the microbial community, resulting in the microbial community rapidly adjusting its structure in response to high salinity (Table 1). It was the adding of brine that initiated the transformation of the microbial community from the koji stage into the Pei stage. The absolute abundance of some genera (Aspergillus, Bacillus, and Weissella) declined in the fermentation due to the nonadaption to the hypertonic environment, while the genera (Staphylococcus and Zygosaccharomyces) with highly competitive ability were enriched in the mid-late stage (Fig. 2). Staphylococcus became the dominant genus at the final fermentation stage due to its strong ability to tolerate sodium chloride. Under high-salinity environmental pressure, the community structure gradually tends to be stable, suggesting the systematic self-domestication of microbial communities (39). Additionally, microbial interaction is also an important factor that underpins the community structure and performance (Fig. 2 and 4B). Compared with pure culture, traditional fermented food is a complicated mixed culture system that utilizes a competition, cooperation, or coordination strategy among microbial communities to achieve an enriched culture of functional microbiota (40). Although there were many discrepancies between the results of microbial interactions from in vitro experiments and the predictions based on in situ patterns, which may be caused by other biotic or abiotic factors, this did not mean that there was no interaction between these microorganisms. These negative or positive interactions implied growth inhibition or promotion (41). Negative interactions may be due to species competition for resources (42) or direct conflict involving secretion of inhibitory compounds (43). Cross-feeding is a pivotal mediator for positive interactions, in which one population benefits from the excreted metabolites of another (44). The reproduction and metabolism of microorganisms (e.g., Aspergillus, Bacillus, and Weissella) in the initial stage of fermentation provided growth and metabolic conditions for the enrichment of other microorganisms (e.g., Staphylococcus and Zygosaccharomyces) in the mid-late stage of fermentation. Besides, metagenomics indicated that microbial communities participated in various metabolism pathways, suggesting that the complementarity of metabolic pathways might be the reason for the cooccurrence of related microbes (Fig. 3C). In general, the succession of microbial communities during fermentation was mainly driven by salinity, as well as by interactions between their constituent microbial populations, and finally reached a balance. In other words, core microbiota can use various strategies to shape the microbiota in their favor, but the selection of a specific strategy would depend on the microenvironment (30).
In this study, we predicted the functional potential of the microbial community and pointed out that five dominant genera (Staphylococcus, Bacillus, Weissella, Aspergillus, and Zygosaccharomyces) may play different key roles in different fermentation stages through correlation analysis and metagenomics (Fig. 2 and 3). However, the lag effect of microbial metabolism or the synergistic effect of multiple strains will lead to the inconsistency between microbial succession and physicochemical metabolism dynamics, and the predicted results could not represent the actual functions of the microbes. Therefore, we isolated core species and evaluated their salinity tolerance, interactions, and metabolic characteristics (Fig. 4). In Asia, A. oryzae and B. subtilis have been widely used as the microbial inoculate for the fermentation of bean-based foods (45). The main function of A. oryzae and B. subtilis is to release various enzymes to hydrolyze proteins and starch of the koji, providing precursors for the growth of bacteria and yeast at the Pei fermentation stage, as previous studies reported (10, 46). In addition, A. oryzae, S. gallinarum, and W. confusa had high producing capacities of organic acids and amino acids (Fig. 4D), which caused acidification and provided unique flavor to the BBP. Simultaneously, the presence of B. subtilis, Weissella, and S. gallinarum helped inhibit the growth of harmful bacteria (B. cereus, E. coli, and S. aureus) (Fig. 4C). It has been well documented that Bacillus, LAB, and Staphylococcus are responsible for producing organic acids or a variety of antimicrobial substances that inhibit the growth of pathogens and spoilage microorganisms (33, 47, 48). With the fermentation of BBP, salinity, moisture content, and acidity changed greatly, leading to significant decreases of the biomass of intolerant microorganisms (A. oryzae, Bacillus, and Weissella) and enzyme activities (Fig. 4A and D). In addition, the adding of brine did not completely inhibit the growth of Staphylococcus and Zygosaccharomyces in the Pei fermentation due to their tolerance to high salinity (Fig. 2A and 4A). As in previous studies, Staphylococcus had a strong ability to endure osmotic stress (28), and several species scattered in Staphylococcus were also reported as the dominant bacteria involved in the production of other hypertonic fermentation foods (6, 49). Coagulase-negative staphylococci (CNS) were identified to be critical for fermentation foods, because they could not only take up the dual role of bioprotection but also generate the customary color and flavor (48, 50). In this study, Staphylococcus gallinarum not only had a certain ability to produce organic acids and amino acids but also inhibited the growth of Staphylococcus aureus (Fig. 4C and D). Staphylococcus is well known for its pathogenicity and is considered a foodborne pathogen, but in fact, most species of Staphylococcus are harmless to humans (51, 52), except S. aureus (53). Whether the presence of Staphylococcus affects safety needs further research. In the mid-late stage, the bacterial community was relative stable (Fig. 2A), while salt-tolerant yeast Zygosaccharomyces began to play its role. Obviously, the biomass of Bacillus and Weissella decreased in the later phase of fermentation, while Zygosaccharomyces increased (Fig. 2F). The existence of Bacillus inhibited the growth of Zygosaccharomyces (Fig. 4B), and the drop of pH by Weissella created the conditions for the growth of Zygosaccharomyces. At higher pH, Z. rouxii is unable to maintain the proton gradient, a key factor affecting its salt tolerance (54). Z. rouxii was mainly responsible for the formation of many volatile flavor components such as alcohols and esters, which directly led to the unique flavor of BBP (Fig. 4D). In general, the complex interactions among microbes and the correlations between microbes and metabolites together define the ultimate taste, aroma, texture, shelf life, and functional properties of fermented foods (55).
As synthetic microbial communities can be reproduced, sampled, and perturbed under well-controlled conditions, they present excellent potential to investigate problems in a complex fermentation ecosystem (56). Previous studies have shown that synthetic microbial communities have broad applications in microbial ecology and biotechnology (13, 57), and most of these processes are performed by the joint efforts of microorganisms with different functions (58). It is generally believed that metabolic potential of the microbial world can be better understood and obtained by exploring metabolic interactions in microbial communities and the ability to stabilize the interactions (59). In this study, taking BBP as a tractable model system, the structure and function were deconstructed and reconstructed in situ and in vitro, which is better to understand the underlying mechanisms of microbial community assembly and microbial function under complex fermentation ecosystem of BBP. Finally, based on microbial interactions and metabolic functions, a synthetic functional microbiota was constructed and realized efficient fermentation of bean-based fermented foods for 6 weeks with low salinity (6%), which presented a highly similar flavor profile to that of products made by using traditional crafts. However, in addition to the active effects of dominant microbes on the ecosystem’s functionality, low-abundance species may be responsible for community resilience and ecosystem function when the environment is altered (60, 61). Therefore, the number of species and the composition, structure, and functionality of synthetic ecosystems should be synthetically optimized in the future, to finally create a synthetic ecosystem with increasing resemblance to the natural ecosystem (58).
MATERIALS AND METHODS
Chemicals and reagents.
High-performance liquid chromatography (HPLC)-grade reagents used for chromatography were obtained from Thermo Fisher Scientific (Waltham, MA, USA). All standard compounds used in the study were from Sigma-Aldrich Co. Ltd. (St. Louis, MO, USA). Other commercial chemicals were of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Sample collection.
All broad bean pastes used in this study were produced by a bean paste factory in Anhui province in China. The Pei was thoroughly mixed once a day during the first week of broad bean paste fermentation and once a week afterwards. Therefore, the Pei was collected on days 0, 1, 2, 3, 4, 5, 6, 13, 20, 27, 34, 41, 48, and 55 of fermentation. As shown in Fig. S1 in the supplemental material, samples (500 g) were taken from the upper, middle, and bottom layers of the same ceramic tank after each pouring and then mixed into one sample. Three different tanks were set as biological duplications. Samples were collected in sterile bottles and stored at −80°C before further analysis.
Physicochemical characteristic analysis.
The moisture content of samples was tested by drying at 105°C. A sample (5 g) was mixed with distilled water (45 ml) and homogenized on a rotary incubator at 100 rpm at 30°C for 1 h. After that, it was centrifuged at 5,000 × g and 4°C for 10 min. Subsequently, the supernatants were collected and filtered prior to assays for salinity, pH, titratable acidity, amino acid nitrogen, protease activity, reducing sugar content, and glucoamylase activity. The salinity was measured by titration with silver nitrate (AgNO3) according to the Mohr method (62). The pH was measured by a pH meter (Mettler Toledo, Shanghai, China). Titratable acidity represented by lactic acid was tested by acid-base titration (63). Protease activity was measured using the Folin-Ciocalteu method (64). The 3,5-dinitrosalicylic acid (DNS) method was utilized to determine the content of reducing sugars and glucoamylase activities (65). All measurements were performed in triplicates.
Seven organic acids (oxalic acid, tartaric acid, pyruvic acid, lactic acid, acetic acid, citric acid, and succinic acid) and seventeen free amino acids (Glu, Arg, Lys, Leu, Val, Phe, Asp, Tyr, Ile, Ala, Thr, His, Pro, Gly, Met, Ser, and Cys-s) were analyzed by HPLC with approaches described by Wu et al. (66). A gas chromatography mass spectrometry method coupled with headspace solid-phase microextraction (HS-SPME/GC-MS) was used to detect the volatile flavor compounds in BBP. The sample (2 g) saturated with NaCl and added to 2-octanol as an internal standard was sealed in a dedicated bottle. Prior to analysis, the samples were preheated in a 55°C water bath for 30 min. The volatiles were adsorbed using the SPME fiber for 40 min, and the concentrated volatiles were desorbed at 250°C in the injection port of gas chromatograph (Trace GC-1310-ISQ LT; Thermo Finnigan, Austin, TX, USA) by holding in the splitless mode for 3 min. Separations of the volatile compounds were performed on a DB-WAX capillary column (30 m by 0.25 mm covered with 0.25-μm film thickness; Agilent Technology, Santa Clara, CA, USA). The initial temperature of the GC oven was held at 40°C for 2 min and then ramped to 230°C at 5°C/min and held for 8 min at final temperature. Helium was used as the carrier gas at the flow rate of 1.2 ml/min. MS was operated in the electron impact (EI) mode with an ion source temperature of 260°C and an ionization voltage of 70 eV. Mass scan range was 25 to 500 atomic mass units (amu) with a scanning rate of 0.2 scan/s. Compound identification was performed by matching the mass spectra with the NIST library and Wiley registry (NY, 320,000 compounds, ver. 6.0). Compound quantification was calculated according to the ratio between the peak area of a particular compound and that of internal standard, 2-octanol.
Amplicon and metagenomic sequencing.
The samples were ground into powder in liquid nitrogen with pestles. Next, the DNA was extracted using the DNeasy Power Max Soil kit (MoBio, Carlsbad, CA, USA). All the extraction steps were performed according to the manufacturer’s instructions. The purity and integrity of extracted DNA were checked by a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis. The DNA was stored at −80°C prior to further analysis.
The V3-V4 region of bacterial 16S rRNA genes and fungal internal transcribed spacer 1 (ITS1) regions were amplified by primers 338-F/806-R and ITS1-F/ITS2-R with specific barcodes, respectively (65). PCR products were purified and quantified according to the methods of Li et al. (67). Finally, the library was sequenced on an Illumina HiSeq 2500 platform by BGI Co., Ltd. (Wuhan, China). After sequencing, the raw sequences were quality filtered and merged according to the methods of Li et al. (68). The effective sequences were clustered into operational taxonomic units (OTUs) at 97% similarity by QIIME (version 1.9.1). The representative OTU sequences were annotated using the Greengenes database (version 13.8) and the UNITE fungal ITS database (version 7.1). Alpha diversity indices of Shannon and beta diversity estimates of Bray-Curtis dissimilarity matrices were calculated within QIIME (version 1.9.1).
The DNA was randomly sheared using a Covaris ultrasonic processor into approximately 400-bp fragments, which were then used to construct the metagenomics sequencing libraries using the NEXTFLEX Rapid DNA-Seq kit (Bioo Scientific, Austin, TX, USA). Sequencing was performed with an Illumina HiSeq 4000 by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads from metagenome sequencing were filtered to obtain clean reads, and then high-quality short reads of each DNA sample were assembled by megahit. The open reading frames (ORFs) were predicted using MetaGene. The nonredundant gene categories (unigenes) were generated using CD-HIT with 95% identity and 90% coverage. Then, the taxonomic annotations and functional annotation were assigned to the NR database and KEGG orthology database using DIAMOND software with an E value of <1 × 105. Taxonomic assignment of predicted genes was carried out using BLASTP alignment against the integrated NR database.
Quantitative real-time PCR.
To quantify the microbial biomass of samples, quantitative real-time PCR (qPCR) was performed with the SYBR Select master mix for CFX (Applied Biosystems, Foster City, CA, USA) on a CFX Connect real-time system (Bio-Rad, Hercules, CA, USA). The primers used for quantitative real-time PCR analysis in our work are shown in Table 2. Each reaction was performed in 20-μl reaction mixtures containing 10 μl SYBR (TaKaRa, Dalian, China), 1 μl of each primer (10 μmol/liter), and 1 μl of DNA template. Gene copy numbers were calculated by comparing sample quantification cycle (Cq) values to a standard curve.
TABLE 2.
Primer sequences and annealing temperatures used for qPCR in this study
| Microbial target | Primers sequence (5′→3′) | Tm (°C) | Reference |
|---|---|---|---|
| Aspergillus | GTCGTCCCCTCTCCGG | 60 | 70 |
| CTGGAAAAAGATTGATTTGCG | |||
| Bacillus | TTGACATCCTCTGACAACCCT | 60 | This study |
| GAATGCTGGCAACTAAGATCA | |||
| Staphylococcus | ACGGTCTTGCTGTCACTTATA | 60 | 71 |
| TACACATATGTTCTTCCCTAATAA | |||
| Weissella | CGTGGGAAACCTACCTCTTAGCAG | 55 | 72 |
| GACCATCTCTTAGTGATAGCAGAACCAT | |||
| Zygosaccharomyces | CCTGTTTGAGCGTCATTTC | 55 | This study |
| GCACAGTCGCAAACTAAGC |
Isolation of bacterial and fungal strains.
For the isolation of microbes, 10 g of fermented Pei was mixed with 90 ml of sterile saline solution (0.85%) and incubated at 200 rpm and 30°C for 1 h. Then, the suspension was serially diluted 10-fold in sterile saline solution, and 100-μl dilutions were spread on the surface of the Luria-Bertani (LB), de Man-Rogosa-Sharpe (MRS), yeast extract-peptone-dextrose (YPD), and potato dextrose agar (PDA) plates and incubated at 30°C and 37°C for 3 days under aerobic and anaerobic conditions. Finally, the single isolated strains were identified according to the methods described in a previous study (69).
Salt tolerance analysis of dominant species.
The salt tolerances of strains (A. oryzae, Z. rouxii, S. gallinarum, B. subtilis, and W. confusa) were determined by assessing growth on suitable medium supplemented with 0%, 4%, 8%, 12%, 16%, and 20% (wt/vol) NaCl after a 3-day incubation. Aspergillus oryzae and Z. rouxii were incubated in PDA and YPD media at 30°C, respectively. S. gallinarum, B. subtilis, and W. confusa were incubated in MRS medium at 37°C. Cell concentration was calculated by absorbance values measured at 600 nm.
Analysis of microbial interactions and function.
Ten grams of broad bean flour, 2.5 g of wheat flour, and 50 g of BBP were added to 1.0 liter of deionized water, steamed for 30 min, and then filtered to make simulated liquid medium. Dominant microbes were then inoculated in liquid medium to simulate the fermentation of BBP, with the initial cell density of each species adjusted to 1 × 106 CFU/g. All the fermentations were conducted without agitation at 30°C for 3 days. A noninoculated sample of fermentation medium was prepared as the control. The cell numbers of different species at the end of fermentation were quantified using quantitative real-time PCR. The enzyme activities, organic acids, amino acids and volatile flavor compounds were analyzed by the method described in “Physicochemical characteristic analysis” above. All experiments were performed in triplicates.
Antimicrobial test.
Antimicrobial testing was conducted by the modified agar diffusion assay (69). Briefly, 6-mm sterile filter paper was put in the middle of an agar plate which had been covered uniformly with 100 μl of the indicative microbe (107 CFU/ml). A 10-μl aliquot of culture broth of the experimental microbe was added to the filter paper. Growth inhibition was verified by the bacteriostatic ring after incubation at 30°C for 48 h.
Reconstruction of microbial community.
Representative species of the dominant genera were selected from the microbes we isolated to design synthetic microbiomes (listed in Table S5). Broad bean (500 g) was soaked in 1 liter of water for 8 h at room temperature and autoclaved for 15 min at 115°C. The broad bean was cooled to room temperature and then mixed thoroughly with the wheat flour (3:1 [wt/wt]). The mixture was inoculated with A. oryzae spores to a final concentration of 106 spores/g substrate and incubated at 30°C for 3 days, resulting in a formation of koji. Koji was then mixed with different concentrations of salted water to obtain 55% water content and 6%, 9%, and 12% salt concentration Pei. Then, logarithmic phase cells of B. subtilis, S. gallinarum, W. confusa, and Z. rouxii were inoculated in the mixed Pei with approximately equal in situ initial ratios and CFUs. The Pei was incubated at 30°C for 5 weeks and mixed every week until the end of fermentation. After fermentation, the Pei were stored at −20°C for analysis of physicochemical metabolites. Each treatment was conducted in triplicates and along with a Pei sample not inoculated with strains as a control.
Statistical analysis.
Principal-component analysis (PCA) was performed with packages ade4 and ggplot2 of R (version R-3.5.1). The Spearman’s pairwise correlations were calculated simultaneously using corr.test function with the psych package in R to analyze the significance of the correlation. Significantly (P value < 0.05) high correlations (|ρ| > 0.6) were visualized via Cytoscape (v.3.6.1). The Mantel test was used to assess the Spearman’s correlation between the dissimilarity matrix of microbial succession and the distance matrix of environmental variables in R (version R-3.5.1) via the vegan package. Redundancy analysis (RDA) was implemented with Canoco 5.0 (Microcomputer Power, Ithaca, NY, USA) according to the manufacturer’s instructions. The ANOSIM test was calculated with packages vegan of R (version R-3.5.1). Further statistical analysis and graphics were performed in Excel 2017 software (Microsoft Office, USA) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA).
Data availability.
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the Beijing Institute of Genomics (BIG) Data Center, Chinese Academy of Sciences, number PRJCA002416 (https://bigd.big.ac.cn/bioproject/browse/PRJCA002416).
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by National Key Research and Development Program of China (no. 2018YFD0400403), the National Science Foundation (no. 31901626, 31571942, and 31601558) and Program of Introducing Talents of Discipline to Universities (no. 111-2-06).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Taormina PJ. 2010. Implications of salt and sodium reduction on microbial food safety. Crit Rev Food Sci Nutr 50:209–227. doi: 10.1080/10408391003626207. [DOI] [PubMed] [Google Scholar]
- 2.Hoang NX, Ferng S, Ting CH, Huang WH, Chiou RYY, Hsu CK. 2016. Optimizing the initial moromi fermentation conditions to improve the quality of soy sauce. Lebenson Wiss Technol 74:242–250. doi: 10.1016/j.lwt.2016.07.049. [DOI] [Google Scholar]
- 3.Brown IJ, Tzoulaki I, Candeias V, Elliott P. 2009. Salt intakes around the world: implications for public health. Int J Epidemiol 38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 4.Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T, Rodriguez-Iturbe B, MacLean PS, Johnson RJ. 2018. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci U S A 115:3138–3143. doi: 10.1073/pnas.1713837115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singracha P, Niamsiri N, Visessanguan W, Lertsiri S, Assavanig A. 2017. Application of lactic acid bacteria and yeasts as starter cultures for reduced-salt soy sauce (moromi) fermentation. Lebenson Wiss Technol 78:181–188. doi: 10.1016/j.lwt.2016.12.019. [DOI] [Google Scholar]
- 6.Yan YZ, Qian YL, Ji FD, Chen JY, Han BZ. 2013. Microbial composition during Chinese soy sauce koji-making based on culture dependent and independent methods. Food Microbiol 34:189–195. doi: 10.1016/j.fm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Watanabe J, Mogi Y. 2012. Monitoring of the microbial communities involved in the soy sauce manufacturing process by PCR-denaturing gradient gel electrophoresis. Food Microbiol 31:100–106. doi: 10.1016/j.fm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim TW, Lee JH, Park MH, Kim HY. 2010. Analysis of bacterial and fungal communities in Japanese- and Chinese-fermented soybean pastes using nested PCR-DGGE. Curr Microbiol 60:315–320. doi: 10.1007/s00284-009-9542-4. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Rui J, Li X, Li J, Dong L, Huang Q, Huang C, Wang Z, Li L, Xuan P, Tang Y, Chen F. 2017. Bacterial community succession and metabolite changes during doubanjiang-meju fermentation, a Chinese traditional fermented broad bean (Vicia faba L.) paste. Food Chem 218:534–542. doi: 10.1016/j.foodchem.2016.09.104. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Tian T, Liu Y, Shi Y, Tao D, Wu R, Yue X. 2018. The dynamic changes of chemical components and microbiota during the natural fermentation process in Da-Jiang, a Chinese popular traditional fermented condiment. Food Res Int 112:457–467. doi: 10.1016/j.foodres.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Vrancken G, Gregory AC, Huys GRB, Faust K, Raes J. 2019. Synthetic ecology of the human gut microbiota. Nat Rev Microbiol 17:754–763. doi: 10.1038/s41579-019-0264-8. [DOI] [PubMed] [Google Scholar]
- 12.Vilanova C, Porcar M. 2016. Are multi-omics enough? Nat Microbiol 1:16101. doi: 10.1038/nmicrobiol.2016.101. [DOI] [PubMed] [Google Scholar]
- 13.Lawson CE, Harcombe WR, Hatzenpichler R, Lindemann SR, Löffler FE, O'Malley MA, García Martín H, Pfleger BF, Raskin L, Venturelli OS, Weissbrodt DG, Noguera DR, McMahon KD. 2019. Common principles and best practices for engineering microbiomes. Nat Rev Microbiol 17:725–741. doi: 10.1038/s41579-019-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe BE, Button JE, Santarelli M, Dutton RJ. 2014. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 158:422–433. doi: 10.1016/j.cell.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Props R, Kerckhof FM, Rubbens P, De Vrieze J, Hernandez Sanabria E, Waegeman W, Monsieurs P, Hammes F, Boon N. 2017. Absolute quantification of microbial taxon abundances. ISME J 11:584–587. doi: 10.1038/ismej.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandeputte D, Kathagen G, D'hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, Wang J, Tito RY, De Commer L, Darzi Y, Vermeire S, Falony G, Raes J. 2017. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Y, Lu X, Xing L, Ho SWA, Kwan HS. 2018. Genomic and transcriptomic comparison of Aspergillus oryzae strains: a case study in soy sauce koji fermentation. J Ind Microbiol Biotechnol 45:839–853. doi: 10.1007/s10295-018-2059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Cui C, Ren J, Zhao H, Zhao Q, Zhao M. 2011. Changes in the chemical composition of traditional Chinese-type soy sauce at different stages of manufacture and its relation to taste. Int J Food Sci Technol 46:243–249. doi: 10.1111/j.1365-2621.2010.02487.x. [DOI] [Google Scholar]
- 19.Kum SJ, Yang SO, Lee SM, Chang PS, Choi YH, Lee JJ, Hurh BS, Kim YS. 2015. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J Agric Food Chem 63:1401–1418. doi: 10.1021/jf5056002. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Tramper J. 2013. Koji–where East meets West in fermentation. Biotechnol Adv 31:1448–1457. doi: 10.1016/j.biotechadv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Kong Y, Zhang L, Zhang Y, Sun B, Sun Y, Zhao J, Chen H. 2018. Evaluation of non-volatile taste components in commercial soy sauces. Int J Food Prop 21:1854–1866. doi: 10.1080/10942912.2018.1497061. [DOI] [Google Scholar]
- 22.Qi W, Guo HL, Wang CL, Hou LH, Cao XH, Liu JF, Lu FP. 2017. Comparative study on fermentation performance in the genome shuffled Candida versatilis and wild-type salt tolerant yeast strain. J Sci Food Agric 97:284–290. doi: 10.1002/jsfa.7728. [DOI] [PubMed] [Google Scholar]
- 23.Harada R, Yuzuki M, Ito K, Shiga K, Bamba T, Fukusaki E. 2018. Microbe participation in aroma production during soy sauce fermentation. J Biosci Bioeng 125:688–694. doi: 10.1016/j.jbiosc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Syifaa AS, Jinap S, Sanny M, Khatib A. 2016. Chemical profiling of different types of soy sauce and the relationship with its sensory attributes. J Food Quality 39:714–725. doi: 10.1111/jfq.12240. [DOI] [Google Scholar]
- 25.Feng Y, Cai Y, Su G, Zhao H, Wang C, Zhao M. 2014. Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chem 145:126–134. doi: 10.1016/j.foodchem.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 26.Huang ZR, Hong JL, Xu JX, Li L, Guo WL, Pan YY, Chen SJ, Bai WD, Rao PF, Ni L, Zhao LN, Liu B, Lv XC. 2018. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiol 76:487–496. doi: 10.1016/j.fm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Qu Y, Li S, Feng K, Wang S, Cai W, Liang Y, Li H, Xu M, Yin H, Deng Y. 2017. Soil bacterial quantification approaches coupling with relative abundances reflecting the changes of taxa. Sci Rep 7:4837. doi: 10.1038/s41598-017-05260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S, Jung J, Jeon CO, Park W. 2014. Comparative genomic and transcriptomic analyses of NaCl-tolerant Staphylococcus sp. OJ82 isolated from fermented seafood. Appl Microbiol Biotechnol 98:807–822. doi: 10.1007/s00253-013-5436-2. [DOI] [PubMed] [Google Scholar]
- 29.Sato A, Matsushima K, Oshima K, Hattori M, Koyama Y. 2017. Draft genome sequencing of the highly halotolerant and allopolyploid yeast Zygosaccharomyces rouxii NBRC 1876. Genome Announc 5:e01610-16. doi: 10.1128/genomeA.01610-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee S, Schlaeppi K, Heijden M. 2018. Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol 16:567–576. doi: 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Wu Q, Nie Y, Wu J, Xu Y. 2019. Construction of synthetic microbiota for reproducible flavor compound metabolism in Chinese light-aroma-type liquor produced by solid-state fermentation. Appl Environ Microbiol 85:e03090-18. doi: 10.1128/AEM.03090-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Chai S, Li Y, Huang J, Luo Y, Xiao L, Liu Z. 2018. Biochemical components associated with microbial community shift during the pile-fermentation of primary dark tea. Front Microbiol 9:1509. doi: 10.3389/fmicb.2018.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eom JS, Lee SY, Choi HS. 2014. Bacillus subtilis HJ18-4 from traditional fermented soybean food inhibits Bacillus cereus growth and toxin-related genes. J Food Sci 79:M2279–M2287. doi: 10.1111/1750-3841.12569. [DOI] [PubMed] [Google Scholar]
- 34.Devanthi PVP, Linforth R, Onyeak H, Gkatzionis K. 2018. Effects of co-inoculation and sequential inoculation of Tetragenococcus halophilus and Zygosaccharomyces rouxii on soy sauce fermentation. Food Chem 240:1–8. doi: 10.1016/j.foodchem.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 35.Gobbetti M. 1998. The sourdough microflora: interactions of lactic acid bacteria and yeasts. Trends Food Sci Technol 9:267–274. doi: 10.1016/S0924-2244(98)00053-3. [DOI] [Google Scholar]
- 36.Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, Fukuda S, Ushio M, Nakaoka S, Onoda Y, Yoshida K, Schlaeppi K, Bai Y, Sugiura R, Ichihashi Y, Minamisawa K, Kiers ET. 2018. Core microbiomes for sustainable agroecosystems. Nat Plants 4:247–257. doi: 10.1038/s41477-018-0139-4. [DOI] [PubMed] [Google Scholar]
- 37.Liu YR, Delgado-Baquerizo M, Bi L, Zhu J, He JZ. 2018. Consistent responses of soil microbial taxonomic and functional attributes to mercury pollution across China. Microbiome 6:183. doi: 10.1186/s40168-018-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nam YD, Lee SY, Lim SI. 2012. Microbial community analysis of Korean soybean pastes by next-generation sequencing. Int J Food Microbiol 155:36–42. doi: 10.1016/j.ijfoodmicro.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Lu ZM, Wang ZM, Zhang XJ, Mao J, Shi JS, Xu ZH. 2018. Microbial ecology of cereal vinegar fermentation: insights for driving the ecosystem function. Curr Opin Biotechnol 49:88–93. doi: 10.1016/j.copbio.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Mu DS, Liang QY, Wang XM, Lu DC, Shi MJ, Chen GJ, Du ZJ. 2018. Metatranscriptomic and comparative genomic insights into resuscitation mechanisms during enrichment culturing. Microbiome 6:230. doi: 10.1186/s40168-018-0613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widder S, Allen RJ, Pfeiffer T, Curtis TP, Wiuf C, Sloan WT, Cordero OX, Brown SP, Momeni B, Shou W, Kettle H, Flint HJ, Haas AF, Laroche B, Kreft JU, Rainey PB, Freilich S, Schuster S, Milferstedt K, van der Meer JR, Grobetakopf T, Huisman J, Free A, Picioreanu C, Quince C, Klapper I, Labarthe S, Smets BF, Wang H, Isaac Newton Institute Fellows, Soyer OS. 2016. Challenges in microbial ecology: building predictive understanding of community function and dynamics. ISME J 10:2557–2568. doi: 10.1038/ismej.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. 2010. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley MA, Wertz JE. 2002. Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol 56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 44.Sieber JR, McInerney MJ, Gunsalus RP. 2012. Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu Rev Microbiol 66:429–452. doi: 10.1146/annurev-micro-090110-102844. [DOI] [PubMed] [Google Scholar]
- 45.Seo HS, Lee S, Singh D, Shin HW, Cho SA, Lee CH. 2018. Untargeted metabolite profiling for koji-fermentative bioprocess unravels the effects of varying substrate types and microbial inocula. Food Chem 266:161–169. doi: 10.1016/j.foodchem.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 46.Furukawa S, Watanabe T, Toyama H, Morinaga Y. 2013. Significance of microbial symbiotic coexistence in traditional fermentation. J Biosci Bioeng 116:533–539. doi: 10.1016/j.jbiosc.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Cappello MS, Zapparoli G, Logrieco A, Bartowsky EJ. 2017. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int J Food Microbiol 243:16–27. doi: 10.1016/j.ijfoodmicro.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Sánchez Mainar M, Xhaferi R, Samapundo S, Devlieghere F, Leroy F. 2016. Opportunities and limitations for the production of safe fermented meats without nitrate and nitrite using an antibacterial Staphylococcus sciuri starter culture. Food Control 69:267–274. doi: 10.1016/j.foodcont.2016.04.056. [DOI] [Google Scholar]
- 49.Jung WY, Jung JY, Lee HJ, Jeon CO. 2016. Functional characterization of bacterial communities responsible for fermentation of doenjang: a traditional Korean fermented soybean paste. Front Microbiol 7:827. doi: 10.3389/fmicb.2016.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stavropoulou DA, De Maere H, Berardo A, Janssens B, Filippou P, De Vuyst L, De Smet S, Leroy F. 2018. Pervasiveness of Staphylococcus carnosus over Staphylococcus xylosus is affected by the level of acidification within a conventional meat starter culture set-up. Int J Food Microbiol 274:60–66. doi: 10.1016/j.ijfoodmicro.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Xie J, Ma X, Huang Y, Mo M, Guo F, Yang Y, Qiu H. 2014. Value of American Thoracic Society guidelines in predicting infection or colonization with multidrug-resistant organisms in critically ill patients. PLoS One 9:e89687. doi: 10.1371/journal.pone.0089687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drosinos EH, Paramithiotis S, Kolovos G, Tsikouras I, Metaxopoulos I. 2007. Phenotypic and technological diversity of lactic acid bacteria and staphylococci isolated from traditionally fermented sausages in Southern Greece. Food Microbiol 24:260–270. doi: 10.1016/j.fm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Jeong DW, Na H, Ryu S, Lee JH. 2016. Complete genome sequence of Staphylococcus equorum KS1039 isolated from Saeu-jeotgal, Korean high-salt-fermented seafood. J Biotechnol 219:88–89. doi: 10.1016/j.jbiotec.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe Y, Tamai Y. 1992. Inhibition of cell growth in Zygosaccharomyces rouxii by proton-ionophore and plasma membrane ATPase inhibitor in the presence of a high concentration of sodium chloride. Biosci Biotechnol Biochem 56:342–343. doi: 10.1271/bbb.56.342. [DOI] [Google Scholar]
- 55.Singh D, Lee S, Lee CH. 2017. Metabolomics for empirical delineation of the traditional Korean fermented foods and beverages. Trends Food Sci Technol 61:103–115. doi: 10.1016/j.tifs.2017.01.001. [DOI] [Google Scholar]
- 56.Wolfe BE, Dutton RJ. 2015. Fermented foods as experimentally tractable microbial ecosystems. Cell 161:49–55. doi: 10.1016/j.cell.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 57.Vorholt JA, Vogel C, Carlstrom CI, Muller DB. 2017. Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 22:142–155. doi: 10.1016/j.chom.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 58.De Roy K, Marzorati M, Van den Abbeele P, Van de Wiele T, Boon N. 2014. Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ Microbiol 16:1472–1481. doi: 10.1111/1462-2920.12343. [DOI] [PubMed] [Google Scholar]
- 59.Grosskopf T, Soyer OS. 2014. Synthetic microbial communities. Curr Opin Microbiol 18:72–77. doi: 10.1016/j.mib.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shade A, Jones SE, Caporaso JG, Handelsman J, Knight R, Fierer N, Gilbert JA. 2014. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 5:e01371-14. doi: 10.1128/mBio.01371-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shade A, Gilbert JA. 2015. Temporal patterns of rarity provide a more complete view of microbial diversity. Trends Microbiol 23:335–340. doi: 10.1016/j.tim.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Al-Mentafji HN. 2005. Official methods of analysis of AOAC International, 18th ed. AOAC International, Gaithersburg, MD. [Google Scholar]
- 63.Jang YK, Shin GR, Jung ES, Lee S, Lee S, Singh D, Jang ES, Shin DJ, Kim HJ, Shin HW, Moon BS, Lee CH. 2017. Process specific differential metabolomes for industrial gochujang types (pepper paste) manufactured using white rice, brown rice, and wheat. Food Chem 234:416–424. doi: 10.1016/j.foodchem.2017.04.154. [DOI] [PubMed] [Google Scholar]
- 64.Gao X, Zhao H, Feng Y, Zhao M. 2010. A comparative study on physicochemical properties of Chinese-type soy sauces prepared using pure koji and mixed kojis. Afr J Biotechnol 9:6740–6747. [Google Scholar]
- 65.Liu Z, Wang Z, Lv X, Zhu X, Chen L, Ni L. 2018. Comparison study of the volatile profiles and microbial communities of Wuyi Qu and Gutian Qu, two major types of traditional fermentation starters of Hong Qu glutinous rice wine. Food Microbiol 69:105–115. doi: 10.1016/j.fm.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 66.Wu LH, Lu ZM, Zhang XJ, Wang ZM, Yu YJ, Shi JS, Xu ZH. 2017. Metagenomics reveals flavour metabolic network of cereal vinegar microbiota. Food Microbiol 62:23–31. doi: 10.1016/j.fm.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Jiang X, Jing Z, Li G, Chen Z, Zhou L, Zhao C, Liu J, Tan Y. 2017. Spatial and seasonal distributions of bacterioplankton in the Pearl River Estuary: the combined effects of riverine inputs, temperature, and phytoplankton. Mar Pollut Bull 125:199–207. doi: 10.1016/j.marpolbul.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 68.Li P, Lin W, Liu X, Wang X, Gan X, Luo L, Lin WT. 2017. Effect of bioaugmented inoculation on microbiota dynamics during solid-state fermentation of Daqu starter using autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol 61:83–92. doi: 10.1016/j.fm.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Du H, Zhang Y, Xu Y. 2017. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation. Appl Environ Microbiol 84:e02369-17. doi: 10.1128/AEM.02369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen B. 2014. Filamentous fungal community structure and interaction between filamentous fungi and yeast associated with Chinese Maotai-flavor liquor fermentation. PhD thesis Jiangnan University, Wuxi, Jiangsu, China. [Google Scholar]
- 71.Matsuda K, Tsuji H, Asahara T, Kado Y, Nomoto K. 2007. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl Environ Microbiol 73:32–39. doi: 10.1128/AEM.01224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de Los Reyes-Gavilán CG, Gueimonde M. 2012. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 79:763–772. doi: 10.1111/j.1574-6941.2011.01261.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the Beijing Institute of Genomics (BIG) Data Center, Chinese Academy of Sciences, number PRJCA002416 (https://bigd.big.ac.cn/bioproject/browse/PRJCA002416).