Highlights
-
•
Nine carotenoids, including phytoene and phytofluene, quantified in red pepper.
-
•
Carotenoid concentrations did not vary significatively after 21 days under refrigeration.
-
•
The most abundant carotenoids in Lamuyo-peppers were capsanthin, β-carotene, lutein and zeaxanthin.
Keywords: Carotenoids, Red pepper, Refrigeration, Storage
Abstract
Red peppers (Capsicum annuum) are rich in carotenoids and are widely grown and consumed all over the world. Today’s consumption patterns are characterized by periodical purchases of food and longer food storage periods, including raw fruits and vegetables, which could have a negative effect on healthy components. This study aims to investigate the individual carotenoid content in Lamuyo-variety red peppers in cool storage (7 °C) for three weeks. Carotenoid concentrations expressed in µg/100g of the edible portion were; lutein (1203), zeaxanthin (853), α-carotene (272), β-carotene (2167), β-cryptoxanthin (525), violaxanthin (770), capsanthin (9667), phytoene (348) and phytofluene (143). Carotenoid concentrations did not significantly vary after 21 days under household refrigeration conditions and thus the nutritional supply of provitamin A carotenoids and of carotenoids with eye health benefits such as lutein and zeaxanthin, as well as others with potential health benefits in humans such as capsanthin, violaxathin, phytoene and phytofluene.
1. Introduction
Peppers are one of the most widely consumed foods throughout the world owing to their attractive colours and strong flavour. Aside from their sensory properties, peppers are also a good source of nutrients and bioactive compounds such as vitamins, carotenoids, anthocyanins, phenolic acids and flavonoids (Hamed, Kalita, Bartolo, & Jayanty, 2019). Of these components, carotenoids in peppers are of special interest owing both to their provitamin A carotenoid content (β-carotene, α-carotene and β-cryptoxanthin) and other carotenoids that are important for human eye health (lutein, zeaxanthin). Other pepper carotenoids also exhibit biological activity in animals and in-vitro studies and are attracting increased attention (for example, neoxanthin inhibits chemically-induced carcinogenesis) (Asai et al., 2004, Sathasivam and Ki, 2018). In addition to their nutritional value, carotenoids act as antioxidants, deactivating free radicals and quenching reactive oxygen species owing to the presence of conjugated double bonds. These carotenoids have been associated with reduced risk of some chronic diseases. For example, α- and β-carotene suppress tumorigenesis in skin, lung, liver and colon; lycopene reduces risk of prostate cancer and cardiovascular disease; lutein and its stereoisomer zeaxanthin (which are components of macular pigment in the eye) reduce the risk of advanced macular degeneration, and apocarotenoids have also shown interesting multifunctional activities and can be useful in the prevention of cancer and other degenerative diseases (Britton et al., 2009, Krinsky and Johnson, 2005, Krinsky et al., 2004, Rodriguez-Concepcion et al., 2018).
In peppers, these different carotenoids are present in the sacrocarps and develop and accumulate quickly as the fruit ripens. Throughout the ripening process, chloroplasts differentiate into chromoplasts containing different carotenoids which contribute collectively to different fruit colors ranging from green to brown then to yellow, orange, red and/or dark red at the final stage of ripening depending on the cultivar (Mohd Hassan, Yusof, Yahaya, Mohd Rozali, & Othman, 2019). Pepper carotenoid composition is complex and varies both qualitatively and quantitatively depending on the variety and color. For example, β-carotene, lutein, capsanthin and capsorubin are common to all varieties. In contrast, β-cryptoxanthin, α-carotene, zeaxanthin, violaxanthin and other less common ones (neoxanthin, anteraxanthin) (Dias et al., 2018, Mohd Hassan et al., 2019), as well as other colorless carotenoids whose potential role is beginning to be studied (such as phytoene or phytofluene), differ depending on variety and color (Meléndez-Martínez, Mapelli-Brahm, Benítez-González, & Stinco, 2015).
The carotenoid content of fruits and vegetables is affected by many factors such as variety, ripeness, climate, geographic site of production, the part of the plant used, environmental conditions during agricultural production, postharvest handling, processing and storage conditions (Pugliese et al., 2014; Rodriguez-Amaya & Kimura, 2004; Rodriguez-Concepcion et al., 2018). Specifically, storage temperature after harvesting of vegetables has a direct impact on their metabolism. Unfavorable storage conditions and prolonged storage have been reported to lead to the degradation of carotenoids in vegetables (Kirigia et al., 2018, Spinardi et al., 2016). Considering that domestic consumers tend to store foods for longer periods of time (Dias, Camões, & Oliveira, 2014), it is important to know whether individual carotenoid concentrations are impacted by storage time. The aim of this paper is to look into the impact of storage time under household conditions on the carotenoid content of red peppers during a period of 3 weeks.
2. Material and methods
2.1. Samples and sampling
The plant material used was Lamuyo-type sweet red peppers (Capsicum annuum L.) were acquired from a local supermarket at commercial maturity stage and from the same lot, and were then stored in a household refrigerator at 85% relative humidity and a temperature of 7 ± 1 °C as an optimum pepper storage conditions (i.e. Barzegar, Fateh, & Razavi, 2018).
Carotenoid concentrations of recently purchased peppers were analyzed at week 0 and again after 1, 2 and 3 weeks under refrigerated conditions. The analysis was conducted on two peppers frozen at −80 °C at each point in time. Prior to carotenoid extraction, each pair of peppers was cut longitudinally, the two halves were then put back together and ground in a domestic blender until a purée consistency was achieved. Three samples of approximately 10 g each of purée were used for the carotenoid analysis. Laboratory operations were performed in dimmed light conditions.
2.2. Chemicals
Lutein (xanthophyll from marigold), zeaxanthin, α- and β-carotene, β-criptoxanthin and phytoene, tocopheryl acetate, trimethylamine, celite, potassium hydroxide (KOH) and sodium chloride (NaCl) were obtained from Sigma Aldrich (Madrid, Spain). Anhydrous sodium sulfate and pyrogallic acid were supplied by Panreac (Barcelona, Spain). Methyl tert-butyl ether (MTBE), methanol (MeOH), ethanol, dichloromethane, petroleum ether and diethyl ether, were obtained from Análisis Vínicos (Spain). MeOH and MTBE were High-Performance Liquid Chromatographic (HPLC) grade.
2.3. Carotenoid extraction
Each pepper sample was placed in a mortar and crushed with a pestle, using acetone to extract the carotenoids (at least three times, until a colorless extract was achieved). In the first extraction, the internal standard (α-tocopheryl acetate), celite and 20 mL of acetone were placed in the mortar and the pepper sample was crushed during 1 min. The mixture was vacuum filtered and the solid residue was collected and re-extracted two more times with fresh extraction solvent under the same conditions. Extracts were combined and then transferred into a separating funnel. Distilled water was added along with 10% NaCl solution and diethyl ether: petroleum ether (50:50). The mixture was shaken vigorously and then set aside for the layers to separate. The upper layer containing carotenoids was collected separately after removing the water and NaCl solution. The organic extract was collected in a beaker and anhydrous sodium sulfate was added little by little while swirling the flask to absorb all aqueous content. This extract was then evaporated to dryness in a rotary vacuum evaporator at 35 °C, dissolved in 25 mL of MeOH: MTBE (50:50), filtered under membrane filtration (0.45 µm pore size) and transferred to a vial. Vials were stored at −20 °C under nitrogen atmosphere until they were analysed by HPLC. This extract had to be diluted (1:4) and re-injected to allow the carotenoid quantification.
2.4. Saponification
A fast saponification protocol previously described (Granado, Olmedilla, Gil-Martinez, & Blanco, 2001) was used to release hydrolysed xanthophyll fatty acid esters. Briefly, 400 µL of the extracted carotenoids from peppers were added to a test tube. The same quantity (400 µL) of pyrogallic acid in ethanol and KOH in methanol was then added and placed in an ultrasonic bath in darkness for 7 min. After that, 800 µL of distilled water and 1600 µL of diethyl ether: petroleum ether (50:50) were added. After vortex for 1 min and centrifuge 3 min at 3500 rpm, the organic phase (supernatant) was transferred to another test tube. This process (from the point at which distilled water and diethyl ether: petroleum ether were added) was repeated 2 more times. The organic matter collected was dried under nitrogen and dissolved in 150 µL of MeOH: MTBE (50:50) in preparation for HPLC analysis.
2.5. High-Performance Liquid Chromatographic – Diode Array (HPLC-DAD) carotenoid analysis.
Carotenoid concentrations were determined by HPLC using a system consisting of a model 600 pump, a Rheodyne injector and a 2998 photodiode array (PDA) detector (Waters, Milford, MA, USA) and a C30 YMC column (5 µm, 250 × 4.6 mm i.d.) (Waters, Wilmington, MA, USA) with a guard column (Aquapore ODS type RP-18). Mobile phase was formed by MeOH with 0.1% trimethylamine (solvent A) and MTBE (solvent B) in a linear gradient. At baseline, 25, 55 and 60 min the ratios of the solvents were 95:5, 70:30, 35:65 and 95:5. The detection was performed at a wavelength of 450 nm for carotenoids, 285 nm for both phytoene and the internal standard and 270 nm for phytofluene. Chromatograms were processed using Empower 2 software (Waters, Milford, MA, USA).
Identification of trans-carotenoids (all-E carotenoids) was based on either the available standards or their retention times and comparison with absorption spectra reported in the literature. Carotenoid quantification was performed using calibration curves for lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, violaxanthin and neoxanthin, with four concentration levels. No standards were available for capsanthin and phytofluene so they were quantified using zeaxanthin and phytoene standards respectively.
The concentrations of the carotenoids in the curve were: 0.75–3.75 ng/µL for lutein (R2 = 0.999), 0.6–3 ng/µL for zeaxanthin (R2 = 0.999), 1.04–5.25 ng/µL for β-criptoxanthin (R2 = 0.997), 0.45–2.25 ng/µL for α-carotene (R2 = 0.998), 1.05–4.2 ng/µL for β-carotene (R2 = 0.980) and 1.15–11.4 ng/µL for violaxanthin (R2 = 0.989). The recoveries of analyzed internal standard were between 85.8% and 117.6%. The precision was evaluated by the relative coefficient of variation (%CV) which ranges from 4.23 (β-carotene) to 4.79 (lutein).
To get an idea of spectral fine structure, % II/III was calculated along with the ƛMAX values. % II/III is the ratio of the height of the longest-wavelength absorption peak, designated as III, and that of the middle absorption peak, designated as II, taking the valley between the two peaks as the baseline multiplied by 100 (Britton, 1995).
The amount of each carotene extracted (α-carotene, β-carotene, phytoene and phytofluene) was calculated on a fresh-weight basis using the following formula:
where the ng of carotenoid is the quantity of each carotenoid calculated from the standard calibration curve in ng, Vr is the volume of reconstitution (25000 µL), DF is the dilution factor (4), Vi is volume of injection (50 µL) and Wt is weight of fresh sample (10 g aprox.).
The amount of each xanthophyll extracted after saponification (lutein, zeaxanthin, β-cryptoxanthin and capsanthin) was calculated on a fresh weight basis using the following formula:
where the ng of carotenoid is the quantity of each carotenoid calculated from the standard calibration curve, Vr is the volume of reconstitution (25000 µL), DF is the dilution factor (4), Vrs is the volume of reconstitution of the saponified sample (150 µL), Vi is volume of injection (50 µL) , Vs is the volume of sample saponified (400 µL) and Wt is the weight of fresh sample (10 g aprox.).
2.6. Statistical analysis
Data refer to analytes in the all-E form and are based on three weighted sample determinations. Results are presented as mean, standard deviation, and median. The Kolmogorov-Smirnov test was used to ascertain whether the variables followed a standard distribution. The Kruskal-Wallis analysis of variance test was used to compare groups and the Bonferroni-corrected Mann-Whitney U test was used to measure significance for multiple comparisons. Associations between each carotenoid and previously published texture parameters [Steady-state force peaks (CSSFP) and Maximum force (VFM)] (Alvarez, Velarde, Barrios, & Herranz, 2020) were established using Spearman’s rho correlation coefficient. All reported P-values are based on a two-sided test and compared to a significance level of 5%. Data were analyzed with IBM SPSS 25.0 (Armonk, NY, USA; IBM Corp).
3. Results
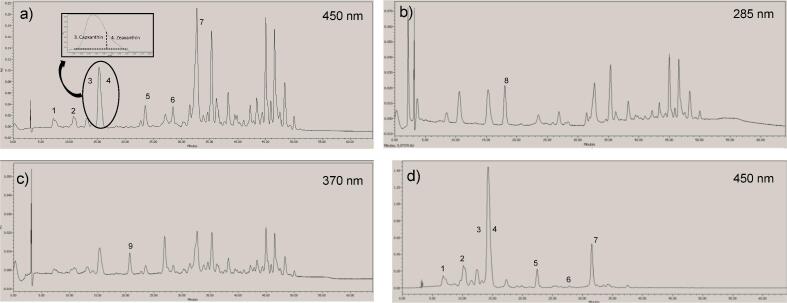
The carotenoids detected and quantified in red Lamuyo-type sweet peppers were β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, violaxanthin, capsanthin, phytoene and phytofluene. Their chromatographic and UV–Vis characteristics in red peppers are shown in Table 1. Chromatograms of the carotenoid extract in the sample at baseline are shown in Fig. 1. Zeaxanthin coelutes with capxanthin at minute 15–16 (Fig. 1), but they could be quantified from their different maximum wavelengths (474.8 and 450.5; 477.3; respectively) (Table 1, Fig. 2). Identification of violaxanthin required concentrating the extract ten-fold because the peak eluting between minutes 7 and 8 and featured an absorption spectrum with three maximum values (411.8; 434.8 and 426.7 nm) which could correspond to violaxanthin (retention time = 7.43 and ƛMAX = 411.8; 438.4 and 467.5 nm), or to neoxanthin (retention time = 8.38 and ƛMAX = 411.8; 434.8 and 463.9 nm). However, in the concentrated sample the spectrum of the peak (retention time = 7.21) gave rise to a spectrum with the following maximum values: 411.8; 440.8 and 470.0 nm. We therefore reached the tentative conclusion that said peak corresponded to violaxanthin.
Table 1.
Chromatographic and UV–Vis characteristics of carotenoids from red pepper obtained by HPLC-DAD.
| Peak | Carotenoid | tR (min) | ƛMAX (nm) | %III/II |
|---|---|---|---|---|
| 1 | Violaxanthin | 7.2 | 411.8; 434.4; 426.7 | 56.5 |
| 2 | Lutein | 10.2 | 444.5;472.4 | 65.2 |
| 3 | Capsanthin | 15.2 | 474.8 | 0 |
| 4 | Zeaxanthin | 14.9 | 450.5;477.3 | 41.0 |
| 5 | β-criptoxanthin | 22.4 | 450;477.3 | 32.8 |
| 6 | α-carotene | 28.4 | 444.5;472.4 | 71.5 |
| 7 | β-carotene | 32.5 | 450.5;477.3 | 45.6 |
| 8 | Phytoene | 17.2 | 284.9 | 0 |
| 9 | Phytofluene | 20.7 | 330;347;363.5 | 86.7 |
Fig. 1.
Chromatogram of non-esterified carotenoids and carotenoid esters at 450 nm (a); 285 nm (b) and 370 nm (c) and chromatogram after saponification at 450 nm (d) at week 0 (beggining of the study).
Fig. 2.
Absorption spectra (450 nm) of capsanthin (a) and zeaxanthin (b) in red peppers.
The carotenoid content in the Lamuyo red peper, at baseline and weekly throughout the three weeks study, is shown in Table 2. The most abundant carotenoid was capsanthin (9667 μg/100 g), followed by β-carotene (2167 μg/100 g), lutein (1203 μg/100 g), zeaxanthin (853.0 μg/100 g) and violaxanthin (770.1 μg/100 g). Small concentrations of β-criptoxanthin (524.6 μg/100 g), phytoene (347.8 μg/100 g), α-carotene (272.1 μg/100 g) and phytofluene (142.9 μg/100 g) were also found. It should be noted that capsanthin and phytofluene were calculated by means of a factor response against a zeaxanthin and phytoene standard respectively and therefore, their concentrations may not be as accurate as those of the other carotenoid quantified.
Table 2.
Carotenoid concentration in red pepper at different weeks of storage in a domestic refrigerator (µg/100 g edible portion, mean ± SD, [median]*).
| Time period | Violaxantin | Lutein | Capsanthin | Zeaxanthin | β-cryptoxanthin | α-carotene | β-carotene | Phytoene | Phytofluene |
|---|---|---|---|---|---|---|---|---|---|
| Baseline (week 0) | 770.1 ± 16.7 [770] |
1202.5 ± 63.3 [1203] |
9667 ± 1584 [9667] |
853.1 ± 65.9 [853.2] |
524.6 ± 4.7 [524.6] |
272.1 ± 67.5 [272] |
2167 ± 249 [2167] |
347.8 ± 8.9 [346.9] |
142.6 ± 2.1 [142.2] |
| Week 1 | 526.5 ± 218.7 [484.3] |
791.4 ± 287.3 [738.4] |
5722 ± 3202 [5414.5] |
897.2 ± 40.0 [902.3] |
376.8 ± 22.3 [384.8] |
107.0 ± 87.7 [107] |
1329 ± 261 [1351.3] |
500.0 ± 24.1 [511.8] |
135.3 ± 34.8 [147] |
| Week 2 | 373.3 ± 49.6 [380.8] |
859.8 ± 77.6 [826.8] |
2641 ± 348 [2473.2] |
753.6 ± 90.0 [746.3] |
243.9 ± 60.3 a [273] |
324.0 ± 18.3 [324] |
982 ± 27 a [975.3] |
258.7 ± 27.2b [245.0] |
60.3 ± 9.5 [64.1] |
| Week 3 | 731.1 ± 42.9 [744.3] |
1013 ± 166.6 [1046] |
7021 ± 1647 [7112.7] |
801.3 ± 52.7 [807.7] |
378.7 ± 72.8 [401.9] |
334.8 ± 57.9 [313.7] |
1692 ± 311 [1698] |
377.2 ± 50.8 [403.8] |
121.3 ± 23.9 [133.6] |
* mean of three data for each carotenoid at each point in time, with the exceptions of those corresponding to xanthophylls α- and β-carotene at baseline.
Values are compared in the same column: a: differences with respect to week 0; b: differences with respect to week 1.
The provitamin A carotenoid content (α, β-carotene y β-cryptoxanthin) of this red pepper is 2832.5 μg/100 g. The contribution of Lamuyo-type sweet red pepper to the vitamin A intake expressed as retinol equivalents (RE) (β-carotene/6 + α-carotene/12 + β-cryptoxanthin/12) is 402 μg/100 g.
The concentration of all of the 9 carotenoids included in the study remained relatively stable after 21 days of refrigerated storage. During the time of the study, some changes were observed in the concentrations of β-cryptoxanthin, β-carotene and phytoene which decreased after 14 days of storage but went back up the following week (week 3) (Table 2). The concentration of each carotenoid in the different weeks with respect to its concentration at time zero is represented in Fig. 3. Only differences in phytoene concentrations between weeks 1 and 2 were found.
Fig. 3.
Ratio between carotenoid concentration at each week and basal concentration.
These same peppers were subjected to a texture study and other physicochemical measurements which were recently published (Alvarez et al., 2020) and from which we have taken texture data to assess the potential relationship between carotenoid content, specifically CSSFP (Steady-state force peaks) and VFM (Maximum force), derived from the cutting test and Volodkevich tests, respectively. VMF declined in the second week and then went back up to values similar to those at the start. In contrast, CSSFP declined slowly (but significantly) through the entire storage period. All the carotenoids, with the exception of lutein, showed a significant correlation to some of the texture parameters (Table 3). The highest correlations were those of phytofluene with both parameters, VMF (rho = 0.741, p = 0.006) and CSSFP (rho = 0,822, p = 0,001) and β-crytoxanthin with CSSFP (rho = 0.763, p = 0.006). Sample moisture was 94.6, 95.6, 97.2 and 97.3% at the beginning of the study and day 7, 14 and 21 respectively.
Table 3.
Statistically significant correlations (Spearman’s rho and (p value) between individual carotenoids and CSSFP (Steady-state force peaks) and VFM (Maximum force)* in red peppers.
| Carotenoid | VMF | CSSFP |
|---|---|---|
| Violaxanthin | 0.644 (0.033) | |
| Capsanthin | 0.698 (0.017) | |
| Zeaxanthin | 0.727 (0.011) | |
| β-cryptoxanthin | 0.763 (0.006) | |
| α-carotene | − 0.767 (0.016) | |
| β-carotene | 0.602 (0.005) | |
| Phytoene | 0.755 (0.005) | |
| Phytofluene | 0.741 (0.006) | 0.822 (0.001) |
* Values obtained from Alvarez et al., 2020).
Given that carotenoid concentration varied with fruit ripeness and that CSSFP is a measure of fruit softening, Table 4 shows the relationship between the concentration of each carotenoid and the corresponding CSSFP value at each point in time (it was calculated dividing the carotenoid concentration values shown in Table 2 by CSSFP). The Table shows a gradual increase in this ratio for all carotenoids over the time of the study, although it is only significant in the cases of phytoene and phytofluene.
Table 4.
Carotenoid changes in red pepper during storage in relation to CSSFP values (*Concentration in µg/100 g).
| Period | CSSFP** | Violaxanthin | Lutein | Capsanthin | Zeaxanthin | β-cryptoxanthin | β-carotene | α-carotene | Phytoene | Phytofluene |
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | 31 ± 4.3 | 24.8 ± 0.5 | 38.8 ± 2.0 | 311.8 ± 51.1 | 27.5 ± 2.1 | 16.9 ± 0.2 | 69.9 ± 8.0 | 8.8 ± 2.8 | 11.2 ± 0.3 | 4.6 ± 0.1 |
| Week 1 | 20 ± 2.5 | 26.3 ± 11.0 | 39.6 ± 14.4 | 286.1 ± 160.1 | 44.9 ± 2.0 | 18.8 ± 11.1 | 66.5 ± 13.0 | 5.4 ± 4.4 | 25.0 ± 1.2 a | 6.8 ± 1.7 |
| Week 2 | 15 ± 1.1 | 24.9 ± 3.3 | 57.3 ± 5.2 | 176.1 ± 23.2 | 50.2 ± 6.0 | 16.3 ± 4.0 | 65.5 ± 1.8 | 21.5 ± 1.2 | 17.3 ± 1.8 | 4.0 ± 0.6 |
| Week 3 | 16 ± 1.2 | 45.7 ± 2.7 | 63.3 ± 10.4 | 438.8 ± 102.9 | 50.1 ± 3.3 | 23.7 ± 4.6 | 105.8 ± 19.5 | 20.9 ± 3.6 | 23.6 ± 3.2 | 7.6 ± 1.5b |
** CSSFP (Steady-state force peaks) values obtained from Alvarez et al., 2020).
*Means and standard deviations of triplicate analyses. Values are compared in the same column. Significance was set at p < 0.05 in line with Bonferroni‐corrected Mann‐Whitney U‐test. a: differences with respect to week 0; b: differences with respect to week 2.
4. Discussion
Nine carotenoids from the Lamuyo sweet red pepper variety were quantified and capsanthin was the one with the highest concentration. Red pepper contains all the carotenoids that are typically studied with regard to diet and human health (lutein, zeaxanthin, α-carotene, β-carotene and β-cryptoxanthin), except lycopene. The other carotenoids quantified were violaxanthin, phytoene and phytofluene whose potential health benefits have received less attention until recent years (Meléndez-Martínez et al., 2017). Carotenoid concentrations found in this red pepper are in the same range reported in other studies and as those compiled in a recent database of carotenoid content in Iberoamerican foods (Dias et al., 2018), in which phytoene and phytofluene were not reported in pepper data. The concentration of phytoene and phytofluene was lower than that reported in a study analyzing red peppers from Spain (Biehler et al., 2012), but in both studies phytoene was present in higher concentrations than phytofluene.
The number one carotenoid in red pepper is capsanthin (Arimboor et al., 2015, Perez-Galvez et al., 2003, Suzuki and Mori, 2003), independent of the different factors affecting carotenoid content in foods such as variety, season, geographic location/climate, ripeness and growing conditions (Maiani et al., 2009). Of the carotenoids found in these peppers, capsanthin concentration is by far the highest even considering the high standard deviations of its concentrations (probably due to the fact that no capsanthin standard was used for its quantification). Capsanthin accumulates in the thylakoid membranes of chromoplasts in the ripe pericarp of red peppers and can account for up to 60% of total carotenoids (Suzuki & Mori, 2003). In this study, capsanthin accounts for 84% of total carotenoids found. Capsanthin has exhibited anti-obesity and insulin sensitizing activity in animals (Jo et al., 2017) and there is growing interest in its possible beneficial effects on humans, such as an inhibitory effect on colon carcinogenesis, photoprotection, among others (Fernández-García et al., 2016, Jo et al., 2017, Mohd Hassan et al., 2019).
As the content of the three provitamin A carotenoids (α-carotene, β-carotene, β-cryptoxanthin) in red pepper was 2832.5 μg/100 g (402 µg RE/100 g), the intake of one pepper/day (180 g) would supply 723.6 µg RE, i.e. 96.5% of the population reference intakes for the Europeans (EFSA, 2017). As the Spanish population’s average consumption of peppers (green and red) is 14.4 g/day (AESAN, 2011), one can conclude that red pepper consumption provides 28.9% of the daily vitamin A requirement in the Spanish diet.
This study assessed the effect that domestic storage for three weeks has on red peppers. No significant variations were observed in terms of carotenoid concentration during that period of time (Table 2). To our knowledge, there are no previous studies on carotenoid concentrations in peppers under domestic storage. It would have been reasonable to expect a decrease in carotenoid concentrations during storage since oxidation significantly contributes to the degradation of carotenoids (Boon et al., 2010, Gao and Kispert, 2003). In general terms, low temperature, oxygen deficiency, decreased humidity and absence of light reduce carotenoid degradation during storage (Ferreira and Rodriguez-Amaya, 2008, Song et al., 2018); in fact, spray-dried spinach juice powder samples stored at 4 °C for 56 days registered a decrease in β-carotene and lutein of 68% and 48%, respectively (Syamila, Gedi, Briars, Ayed, & Gray, 2019). In view of the results, we would note that conclusions would have varied if the study had concluded in 14 days. However, storage time was prolonged to obtain the longest storage without any visually perceived alterations in the fruit (such as the presence of mould), i.e. for 21 days. To obtain peppers with a higher nutritional value, longer storage time in the refrigerator is recommended. An increase in ascorbate content in fresh green and red peppers (Capsicum annuum L., variety California) was described for their ripe green and red stages stored for 19 days at 20° C. In other words, longer storage time added extra nutritional value to these peppers (Jiménez, Romojaro, Gómez, Llanos, & Sevilla, 2003). In contrast, in this study red pepper carotenoid concentrations when stored at 7 °C, the ideal temperature for pepper storage (Barzegar et al., 2018) and which is close to domestic storage (in the vicinity of 4 °C), did not show any variation throughout the three weeks of storage. It is worth mentioning, however, that a decline was observed in β-carotene, β-cryptoxanthin and phytoene at 14 days but then these levels subsequently returned to base concentrations. This behavior coincides with a decrease in the VFM value observed for these red peppers at 14 days and their subsequent recovery at the end of the study (Alvarez et al., 2020). In a previous study which focused on the texture of these peppers, CSSFP gradually decreased over the 21 days resulting in a gradual loss of crunchiness during storage (Alvarez et al., 2020) also possibly due to the destruction of the cell structure attributable to an increase of cellulase activity in pepper fruit during storage (Rao, Gol, & Shah, 2011). Furthermore, in the above-mentioned study (Alvarez et al., 2020), total water content increased significantly at weeks 2 and 3 (97.2 and 97.3%, respectively) when compared with weeks 0 and 1 (94.6 and 95.6%, respectively). This result was ascribed to water loss through transpiration, likely also resulting in a fruit weight loss causing an increase in cell wall plasticity, and could indicate a possible increase in cell wall material stiffness by increased internal turgor after 1 week in storage, as the depolymerization and degradation of cell wall constituents had probably not yet begun. At the end of the 3 weeks of conservation, the concentration of all carotenoids in relation to CSSFP values increased slightly (Table 4). This suggests that the concentration of carotenoids that can be extracted is conditioned by the softening or loss of texture in the fruits analysed since regardless of the texture value, they seem to have been extracted more efficiently at the end of the storage period suggesting that carotenoids concentration increased slightly during those 3 weeks.
It is worth mentioning that when changes in the cell wall begin, they are associated with oxidative and hydrolytic processes (Payasi, Nath, Soares, & Singh, 2009). In this connection, changes in the activity of the catalase enzyme were observed from day 15 to 21 in plants stored in cold (between 0 and 6 °C) (Romero-Tejeda, Martínez-Damián, Rodríguez-Pérez, 2015), and from day 7 to 19 in fresh red peppers (Capsicum annuum L., California variety) stored at 20 °C for 19 days (Jiménez et al., 2003), suggesting that as from a certain point in time there is an increase in the production of natural free radicals in stored plants which promotes an antioxidant response. Similarly, other studies on plant foods such as strawberry and peppers also described an increase in the concentration of antioxidants after a period of time in cold storage. For instance, in strawberry stored at different temperatures (0, 5 and 10 °C) during 14 days postharvest, anthocyanin content decreased at 0 °C and 5 °C during the first 5 days, but then increased from that moment onwards (Ayala-Zavala, Wang, Wang, & Gonzalez-Aguilar, 2004). In sweet pepper (Capsicum annuum L.) stored for 21 days at 5 °C, total phenolic compounds tended to increase slightly after 7 days of storage in the control group (Cuadra-Crespo & del Amor, 2010). According to the above-mentioned results, in this study, the concentration of some carotenoids decreased up to week 2 and then increased from the second to the third week of the study, possibly owing to antioxidant activity.
5. Conclusion
This study shows that the conservation of red peppers in household refrigeration at 7 °C for three weeks does not affect carotenoid concentrations and hence the nutritional supply of provitamin A carotenoids and of carotenoids with ocular health benefits such as lutein and zeaxanthin, and of others with potential health benefits for humans such as capsanthin (Mohd Hassan et al., 2019), violaxathin (Pasquet et al., 2011), phytoene and phytofluene (Havas et al., 2018, Meléndez-Martínez et al., 2017).
CRediT authorship contribution statement
Elena Rodríguez-Rodríguez: Investigation, Data curation, Writing - original draft. Milagros Sánchez-Prieto: Investigation. Begoña Olmedilla-Alonso: Conceptualization, Investigation, Methodology, Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank María Dolores Álvarez and Beatriz Herranz for supplying peppers and individual data on texture parameters (J. Texture Stud. 2019; 1-14) and, Laura Barrrios for statistical analysis support. Begoña Olmedilla-Alonso belongs to the Spanish Carotenoid Network (CaRed) funded by the Spanish MINECO (Ministry of Economy, Industry and Competitiveness) grant BIO2015-71703-REDT and the European Carotenoid Network (EuroCaroten) funded by the European Commission COST Action CA15136.
Contributor Information
Elena Rodríguez-Rodríguez, Email: elerodri@ucm.es.
Begoña Olmedilla-Alonso, Email: BOlmedilla@ictan.csic.es.
References
- AESAN (Agencia Española de Seguridad Alimentaria y Nutrición). Datos de consumo de alimentos (gramos/día) a partir del estudio ENIDE, 2011. URL http://www.aesan.mspsi.gob.es/AESAN/docs/docs/evaluacion_riesgos/datos_consumo/ENIDE.pdf. Accessed 08.01.20.
- Alvarez M.D., Velarde C., Barrios L., Herranz B. Understanding the crispy-crunchy texture of raw red pepper and its change with storage time. Journal of Texture Studies. 2020;51:120–133. doi: 10.1111/jtxs.12443. [DOI] [PubMed] [Google Scholar]
- Arimboor R., Natarajan R.B., Menon K.R., Chandrasekhar L.P., Moorkoth V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—a review. Journal of Food Science and Technology. 2015;52:1258–1271. doi: 10.1007/s13197-014-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A., Terasaki M., Nagao A. An epoxide-furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro: Formation and cytostatic activity of neochrome stereoisomers. Journal of Nutrition. 2004;134:2237–2243. doi: 10.1093/jn/134.9.2237. [DOI] [PubMed] [Google Scholar]
- Ayala-Zavala J.F., Wang S.Y., Wang C.Y., Gonzalez-Aguilar G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT – Food Science and Technology. 2004;37:687–695. doi: 10.1016/j.lwt.2004.03.00. [DOI] [Google Scholar]
- Barzegar T., Fateh M., Razavi F. Enhancement of postharvest sensory quality and antioxidant capacity of sweet pepper fruits by foliar applying calcium lactate and ascorbic acid. Scientia Horticulturae. 2018;241:293–303. [Google Scholar]
- Biehler E., Alkerwi A., Hoffmann L., Krause E., Guillaume M., Lair M.L. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J Journal of Food Composition and Analysis. 2012;25:56–65. [Google Scholar]
- Boon C.S., McClements D.J., Weiss J., Decker E.A. Factors influencing the chemical stability of carotenoids in foods. Critical Reviews in Food Science and Nutrition. 2010;50:515–532. doi: 10.1080/10408390802565889. [DOI] [PubMed] [Google Scholar]
- Britton G. UV/visible spectroscopy. In: Britton G., Liaaen-Jensen S., Pfander H., editors. Carotenoids: Spectroscopy. Birkhäuser Verlag; Basel: 1995. pp. 13–63. [Google Scholar]
- Britton, G., Liaaen-Jensen, S., & Pfander, H. (2009). Carotenoids Volume 5: Nutrition and Health. Switzerland: Birkhäuser; Basel.
- Cuadra-Crespo P., del Amor F.M. Effects of postharvest treatments on fruit quality of sweet pepper at low temperature. Journal of Science Food and Agriculture. 2010;90(15):2716–2722. doi: 10.1002/jsfa.4147. [DOI] [PubMed] [Google Scholar]
- Dias M.G., Camões M.F., Oliveira L. Carotenoid stability in fruits, vegetables and working standards – Effect of storage temperature and time. Food Chemistry. 2014;156:37–41. doi: 10.1016/j.foodchem.2014.01.050. [DOI] [PubMed] [Google Scholar]
- Dias M.G., Olmedilla-Alonso B., Hornero-Méndez D., Mercadante A.Z., Osorio C., Vargas-Murga L. Comprehensive database of carotenoid contents in ibero-american foods. A valuable tool in the context of functional foods and the establishment of recommended intakes of bioactives. Journal of Agricultural and Food Chemistry. 2018;66:5055–5107. doi: 10.1021/acs.jafc.7b06148. Epub 2018 May 15. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) (2017). Dietary Reference Values for nutrients. Summary Report. EFSA supporting publication 2017:e15121. doi:10.2903/sp.efsa.2017.e15121.
- Fernández-García E., Carvajal-Lérida I., Pérez-Gálvez A. Carotenoids exclusively synthesized in red pepper (capsanthin and capsorubin) protect human dermal fibroblasts against UVB induced DNA damage. Journal of Photochemistry and Photobiology Sciences. 2016;15:1204–1211. doi: 10.1039/c6pp00134c. [DOI] [PubMed] [Google Scholar]
- Ferreira J.E.M., Rodriguez-Amaya D.B. Degradation of lycopene and b-carotene in model systems and in lyophilized Guava during ambient storage: Kinetics, structure, and matrix effects. Journal of Food Science. 2008;73(8):C589eC594. doi: 10.1111/j.1750-3841.2008.00919.x. [DOI] [PubMed] [Google Scholar]
- Gao Y., Kispert L.D. Reaction of carotenoids and ferric chloride: Equilibria, isomerization, and products. The Journal of Physical Chemistry B. 2003;107:5333–5338. [Google Scholar]
- Granado F., Olmedilla B., Gil-Martinez E., Blanco I.A. Fast, reliable and low-cost saponification protocol for analysis of carotenoids in vegetables. Journal of Food Composition and Analysis. 2001;14:479–489. [Google Scholar]
- Hamed M., Kalita D., Bartolo M.E., Jayanty S.S. Capsaicinoids, polyphenols and antioxidant activities of capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants (Basel) 2019;8:E364. doi: 10.3390/antiox8090364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havas F., Krispin S., Meléndez-Martínez A.J., von Oppen-Bezalel L. Preliminary data on the safety of phytoene- and phytofluene-rich products for human use including topical application. Journal of Toxicology. 2018;5475784:2018. doi: 10.1155/2018/5475784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A., Romojaro F., Gómez J.M., Llanos M.R., Sevilla F. Antioxidant systems and their relationship with the response of pepper fruits to storage at 20 °C. Journal of Agricultural and Food Chemistry. 2003;51:6293–6299. doi: 10.1021/jf030052i. [DOI] [PubMed] [Google Scholar]
- Jo S.J., Kim J.W., Choi H.O., Kim J.H., Kim H.J., Woo S.H. Capsanthin inhibits both adipogenesis in 3T3-L1 preadipocytes and weight gain in high-fat diet-induced obese mice. Biomolecules & Therapeutics. 2017;25:329–336. doi: 10.4062/biomolther.2017.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirigia, D., Winkelmann, T., Kasili, R., & Mibus, H. (2018). Development stage, storage temperature and storage duration influence phytonutrient content in cowpea (Vigna unguiculata L. Walp.). Heliyon, 20, e00656. doi: 10.1016/j.heliyon.2018.e00656. eCollection 2018 Jun. [DOI] [PMC free article] [PubMed]
- Krinsky N., Mayne S.T., Sies H. Marcel Dekker; New York, NY, USA: 2004. Carotenoids in health and disease. [Google Scholar]
- Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Molecular Aspects of Medicine. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Maiani G., Castón M.J., Catasta G., Toti E., Cambrodón I.G., Bysted A. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Molecular Nutrition & Food Research. 2009;53:S194–218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- Meléndez-Martínez A.J., Mapelli-Brahm P., Benítez-González A., Stinco C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Archives of Biochemistry and Biophysics. 2015;572:188–200. doi: 10.1016/j.abb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Meléndez-Martínez A.J., Pérez-Gálvez A., Roca M., Estévez Santiago R., Olmedilla Alonso B., Mercadante A.Z., et al. (2017). Biodisponibilidad de carotenoides, factores que la determinan y métodos de estimación. In: A.J., Meléndez-Martínez (Ed.), Carotenoides en Agroalimentación y Salud (pp. 574–608). México: Terracota.
- Mohd Hassan N., Yusof N.A., Yahaya A.F., Mohd Rozali N.N., Othman R. Carotenoids of capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants. 2019;8(10):469. doi: 10.3390/antiox8100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet V., Morisset P., Ihammouine S., Chepied A., Aumailley L., Berard J.B. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Marine Drugs. 2011;9:819–831. doi: 10.3390/md9050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payasi A., Nath N., Soares A.L., Singh R. Biochemistry of fruit softening: An overview. Physiology and Molecular Biology of Plants. 2009;15:103–113. doi: 10.1007/s12298-009-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Galvez A., Martin H.D., Sies H., Stahl W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. British Journal of Nutrition. 2003;89:787–793. doi: 10.1079/BJN2003842. [DOI] [PubMed] [Google Scholar]
- Pugliese A., O’Callaghan Y., Tundis R., Galvin K., Menichini F., O’Brien N. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum) European Journal of Nutrition. 2014;53:501–510. doi: 10.1007/s00394-013-0555-1. [DOI] [PubMed] [Google Scholar]
- Rao T.V.R., Gol N.B., Shah K.K. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annum L.) Scientia Horticulturae. 2011;132:18–26. [Google Scholar]
- Rodriguez-Amaya D.B., Kimura M. HarvestPlus Technical Monograph. International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT); Washington, DCCali: 2004. Harvest-Plus handbook for carotenoid analysis. [Google Scholar]
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Méndez D.…Olmedilla-Alonso B. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Romero-Tejeda M., Martínez-Damián M., Rodríguez-Pérez J. Effect of storage temperature on enzyme activity and antioxidant capacity in Salvia officinalis L Shoots. Revista Chapingo Serie Horticultura. 2015;XXI:199–213. doi: 10.5154/r.rchsh.2015.01.003. [DOI] [Google Scholar]
- Sathasivam R., Ki J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs. 2018;16(1):26. doi: 10.3390/md16010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Wei Q., Wang X., Li D., Liu C., Zhang M. Degradation of carotenoids in dehydrated pumpkins as affected by different storage conditions. Food Research International. 2018;107:130–136. doi: 10.1016/j.foodres.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Spinardi A., Ferrante A., Spinardi A., Ferrante A. Effect of storage temperature on quality changes of minimally processed baby lettuce. Journal of Food Agriculture and Environment. 2016;10:38–42. [Google Scholar]
- Suzuki K., Mori M. Carotenoid composition of new cultivar of Capsicum annuum during maturation and its high capsanthin content. Journal of The Japanese Society For Food Science and Technology. 2003;50:324–326. [Google Scholar]
- Syamila M., Gedi M.A., Briars R., Ayed C., Gray D.A. Effect of temperature, oxygen and light on the degradation of β-carotene, lutein and α-tocopherol in spray-dried spinach juice powder during storage. Food Chemistry. 2019;284:188–197. doi: 10.1016/j.foodchem.2019.01.055. [DOI] [PubMed] [Google Scholar]