Coronavirus Disease 2019 (COVID‐19) is a fatal disease that could lead to a serious respiratory illness. The World Health Organization (WHO) declared the disease, a global pandemic on March 11, 2020, even as COVID‐19 rapidly spread across the world. According to the 2019 WHO data, an estimated 1.13 billion people suffer from hypertension. Most of the hypertensive patients are assisted by angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) or both. Some patients with cardiac mortality are additionally managed with other classes of combinational therapy. These drugs improve the cardiac profile through an increase in ACE‐2 expression. Moreover, the level of ACE‐2 predominantly available in lung epithelial tissue is an indication of the protective role in respiratory distress syndrome and acute lung injury. 1 , 2 There is plenty of evidence to suggest that ACE2 converts Ang I to Ang‐(1‐9) in the body and Ang II which is converted to Ang‐(1‐7). ACE2 is further thought to be an essential peptide that binds to the Mas receptors in the RAS cascade 3 (Figure 1).
FIGURE 1.
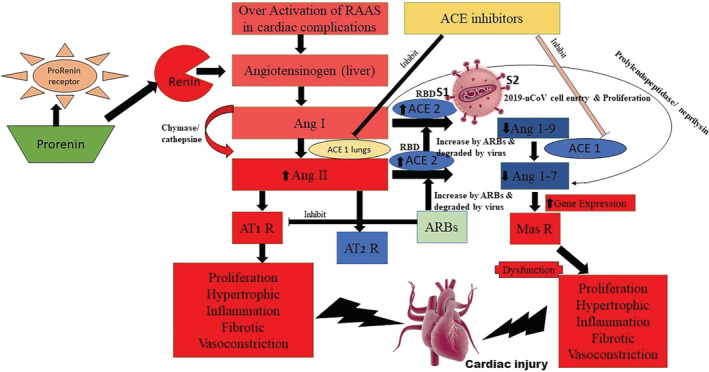
Hypertensive patients assisted with angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) and cell entry of COVID‐19 mediated pathophysiological disturbance and cardiac injury
A recently published study suggested that COVID‐19 also uses ACE2 as a cellular entry receptor, as this was detected in the isolates of the bronchoalveolar lavage fluid of one of the critically ill patients. 4 Moreover, this was further confirmed by another recent study from China, which reported their findings on virus infectivity studies using HeLa cells that were both, expressing and not expressing ACE2 proteins in humans, Chinese horseshoe bats, civets, pigs, and mice. We believe that COVID‐19 is capable of using all, but mouse, ACE2, as a receptor to enter within the cells that express ACE2, but not in cells that lack ACE2, suggesting ACE2 as the most likely cell receptor for COVID‐19. 5 , 6 The SARS‐CoV, which is genetically homologous to COVID‐19, have spikes that are composed of trimers of S glycoprotein that are further cleaved into S1 and S2 subunits by cathepsin L proteases. A fragment located within the S1 subunit, which spans the amino acids 318 to 510, is believed to be the minimal receptor‐binding domain (RBD) complexed with its receptor ACE‐2. 7 , 8 This is the primary component responsible for the binding, with the peptidase domain of ACE2, eventually resulting in enhanced human ACE2‐binding affinity of the COVID‐19. There is enough evidence to believe that the Zoonotic COVID‐19 is completely dependent on human ACE2 as a receptor for entry, thus having high replication potential in human cells. Thus, patients who adhere to RAAS blockers (assisted with ACEi or ARBs) are believed to have a higher risk toward the deadly viral attack of COVID‐19, and progressively they must be switched on to other class of antihypertensive drugs.
REFERENCES
- 1. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imai Y, Kuba K, Penninger JM. Angiotensin‐converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007;64(15):2006‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agata J, Ura N, Yoshida H, et al. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin‐converting enzyme. Hypertens Res. 2006;29(11):865‐874. [DOI] [PubMed] [Google Scholar]
- 4. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. New Engl J Med. 2019;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou P, Yang X‐L, Wang X‐G, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020. 10.1038/s41586-020-2012-7. [DOI] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science (New York, NY). 2005;309(5742):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 8. Prabakaran P, Gan J, Feng Y, et al. Structure of severe acute respiratory syndrome coronavirus receptor‐binding domain complexed with neutralizing antibody. J Biol Chem. 2006;281(23):15829‐15836. [DOI] [PMC free article] [PubMed] [Google Scholar]


