Abstract
SARS‐CoV‐2, a novel emerging coronavirus, has caused severe disease (COVID‐19), and rapidly spread worldwide since the beginning of 2020. SARS‐CoV‐2 mainly spreads by coughing, sneezing, droplet inhalation, and contact. SARS‐CoV‐2 has been detected in saliva samples, making saliva a potential transmission route for COVID‐19. The participants in dental practice confront a particular risk of SARS‐CoV‐2 infection due to close contact with the patients and potential exposure to saliva‐contaminated droplets and aerosols generated during dental procedures. In addition, saliva‐contaminated surfaces could lead to potential cross‐infection. Hence, the control of saliva‐related transmission in the dental clinic is critical, particularly in the epidemic period of COVID‐19. Based on our experience of the COVID‐19 epidemic, some protective measures that can help reduce the risk of saliva‐related transmission are suggested, in order to avoid the potential spread of SARS‐CoV‐2 among patients, visitors, and dental practitioners.
Keywords: clinical practice guidelines, dental public health, epidemiology, infection control, infectious disease(s), oral medicine
SARS‐CoV‐2, a novel emerging coronavirus, has caused severe diseases (COVID‐19), and rapidly spread worldwide since the beginning of 2020. SARS‐CoV‐2 mainly spreads by cough, sneeze, droplet inhalation, and contact. SARS‐CoV‐2 has been detected in saliva samples, making saliva a potential transmission route for COVID‐19. Based on our experience of fighting against the COVID‐19 epidemic, some protective measures that can help reduce the risk of saliva‐related transmission are suggested, in order to avoid the potential spread of SARS‐CoV‐2 among patients, visitors, and dental practitioners.
1. INTRODUCTION
The epidemic of coronavirus disease 2019 (COVID‐19), caused by a novel coronavirus (SARS‐CoV‐2), has become a major public health challenge around the world. In many cases, the infection of SARS‐CoV‐2 resulted from rapid human‐to‐human transmission, including direct contact with patients, and the transmission through droplets and aerosol particles (Xu et al., 2020). The respiratory syndrome caused by this virus is severe and even fatal (Li et al., 2020). It has been reported that angiotensin‐converting enzyme 2 (ACE2) is the main host cell receptor of SARS‐CoV‐2 and plays a crucial role in the entry of virus into the cell (Zhou et al., 2020). Interestingly, the results from our laboratory show that ACE2 is highly expressed on the epithelial cells of oral mucosa, suggesting that the oral cavity could be at high risk for SARS‐CoV‐2 infection, and transmission‐based precautions should be taken in the dental clinic (Xu et al., 2020). Since many viruses including SARS‐CoV‐2 can be detected in saliva (Kaczor‐Urbanowicz et al., 2017; To et al., 2020), the risk of transmission of viruses, especially those that cause respiratory infections, through saliva is non‐negligible in the dental clinic (Meng, Hua, & Bian, 2020). Therefore, based on our experience of fighting against the COVID‐19 epidemic, preventing the spread of disease through saliva in the dental clinic is critical to the health of both doctors and patients (Peng et al., 2020).
2. PHYSIOLOGICAL ATTRIBUTES OF SALIVA
Human saliva is a unique body fluid secreted by the salivary glands. It is mainly composed of water (94%–99%), with organic molecules accounting for approximately 0.5% and inorganic molecules for 0.2% (Zhang et al., 2016). It has the functions of lubricating oral mucosa, digesting food, cleaning and protecting the oral cavity, and is one of the most important factors affecting homeostasis of the oral cavity (Helmerhorst & Oppenheim, 2007). A normal adult secretes approximately 600 ml of saliva every day (Zhang et al., 2016). In addition to salivary gland excreta, saliva also contains food residue, serum components, oral microorganisms, and their metabolites, exfoliated epithelial cells and white blood cells (Proctor et al., 2018).
3. BIOLOGICAL EFFECTS OF SALIVA ON MICROORGANISMS
So far, more than 700 microbial species, many of which are related to oral and systemic diseases, have been identified in saliva. Saliva not only provides ecological niche for the colonization and growth of oral microorganisms, but also inhibits the over‐growth of specific pathogens to maintain the homeostasis of oral cavity.
The salivary acquired pellicle that consist of salivary proteins such as acid proline‐rich protein, statherin and histatins, allow the oral bacteria to colonize on tooth surfaces, and provide nutrition for their survival, reproduction and metabolism (Kolenbrander, 2011). Salivary mucins and glycosylated proteins can provide carbon and nitrogen sources for the growth and metabolism of oral microbiota. The binding between salivary proteins and oral microbes can not only help microbial adherence, but also cause the aggregation of oral microbiota and thus increase the clearance of bacteria from the oral cavity, including some pathogens. Therefore, saliva can act as a “gatekeeper”, and help prevent the spread of pathogens to the respiratory and gastrointestinal tracts (Ruhl, 2012).
In addition to providing a nutritional source for the growth of microorganisms, saliva contains a large number of antibacterial and antiviral proteins. The major antimicrobial components that have been identified in saliva include lysozyme, peroxidase, lactoferrin, and histatins (Tenovuo, 2002). Secretory IgA (sIgA) plays an important role in immunity by binding the surface molecules of pathogenic microorganisms and preventing adhesion and colonization (Balmaseda et al., 2003). Of interest, most of these antibacterial proteins, including cathelcidin (LL‐37), lactoferrin, lysozyme, mucins, peroxidase, salivary agglutinin (gp340, DMBT1), sIgA, SLPI, and α, β defensins, also display antiviral activities, typically against specific viral pathogens (Malamud et al., 2011). The mechanisms of viral inactivation include not only direct binding to the virus, but also indirect methods via intracellular modulation of viral replication, modulation of signaling pathways, and recruitment of immune cells that contribute to antiviral activity in vivo (Diamond, Beckloff, & Ryan, 2008).
4. SALIVA AS A POTENTIAL SOURCE OF VIRUS SPREAD
The interaction between viruses and saliva is a complex biological process. Human saliva is abundant of biologically active components, such as proline‐rich proteins, mucins MG1 and MG2, and gp340. These components interact with pathogens and cause multiple influences on their biological behavior (de Almeida Pdel, Gregio, Machado, de Lima, & Azevedo, 2008). Moreover, pathogenic viruses in saliva have altered biological patterns such as increased aggregation and communicability (Anschau & Sanjuan, 2020). According to a previous study on vesicular stomatitis virus, this pathogen has a strong aggregating ability in the presence of saliva, and some host factors, such as fibrinogen, could promote this saliva‐induced aggregation process, suggesting the important role saliva plays in the biological behavior of this virus (Anschau & Sanjuan, 2020). According to another study, the biochemical components of saliva are closely associated with the presence of Zika virus (ZIKV), indicating complex interactions between the virus and saliva (Siqueira, Moffa, Mussi, & Machado, 2016). Hence investigation of the biological functions of saliva on the behavior of viruses, and the impact of virus on the composition of saliva, suggest the transmission of virus can be closely connected with saliva.
4.1. Spreading of coronavirus through saliva
Coronavirus is a group of enveloped single‐stranded RNA viruses belonging to the order Nidovirales, the coronavirus family, and the coronavirus subfamily. It has 26 known species and can be divided into four genera (α, β, γ, and δ). Only the α and β genus are human pathogenic strains. SARS‐CoV, SARS‐CoV‐2 and the Middle East respiratory syndrome (MERS) coronavirus (MERS‐CoV) all belong to the β subgroup. Studies have shown that early target cells for SARS‐CoV infection include ACE2‐positive cells/keratin epithelial cells in the salivary gland duct and other cells in the lungs, such as ACE2‐positive cells/keratin alveolar epithelial cells, which suggested the salivary gland epithelial cells may be infected in vivo after entry of the virus (Liu et al., 2011). Therefore, the saliva produced by the infected salivary glands could be an important source of virus, particularly in early infection (Liu et al., 2011). Study of the invading process of SARS‐CoV‐2 revealed this pathogen invades human cells through ACE2 which functions as a cell receptor. This process is similar to the invasion process of SARS‐CoV, suggesting a similar invasion mechanism of these two species of coronavirus. Therefore, salivary glands may also become a potential source of the transmission of SARS‐CoV‐2, which is not to be neglected (Wan, Shang, Graham, Baric, & Li, 2020).
Real‐time RT‐PCR detection results of throat wash and saliva showed that the content of SARS‐CoV RNA in saliva was higher than that in throat wash, which supported the possibility of oral droplet transmission of SARS‐CoV (Wang et al., 2004). Studies have found viral RNA and live (culturable) viruses in air samples, and SARS‐CoV may be an opportunistic airborne pathogen (Booth et al., 2005; Xiao et al., 2004). As a result, the virus has the potential to initiate disease through both short‐distance and long‐distance aerosol transmission, and there is at high risk of infection in people who have close and unprotected contact with SARS patients (Tuan et al., 2007). Evidence suggests that aerosols may be produced during endotracheal intubation or in combination with other procedures such as cardiopulmonary resuscitation or bronchoscopy, to increase the risk of SARS transmission (Seto, 2015). To et al reported that SARS‐CoV‐2 can be detected in 91.7% of the saliva samples studied, indicating that saliva as a potential source of SARS‐CoV‐2 spreading (To et al., 2020). Therefore, dental clinicians in close contact with patients, salivary aerosols and blood need to be highly protected to reduce the risk of infection, particularly during the epidemic period of COVID‐19.
4.2. Other viruses
Apart from coronavirus, human saliva also plays a role in the pathogenicity of other viruses. At present, a variety of viruses related to human diseases have been isolated from, or detected in, saliva, including Epstein–Barr virus (Guidry, Birdwell, & Scott, 2018), herpes simplex virus (Corstjens, Abrams, & Malamud, 2016), hepatitis A, B, and C viruses (Mahboobi, Porter, Karayiannis, & Alavian, 2012), cytomegalovirus (Plosa, Esbenshade, Fuller, & Weitkamp, 2012), human papilloma virus (Wang et al., 2015), human immunodeficiency virus (Corstjens et al., 2016), Chikungunya virus (Musso et al., 2016), ZIKV (Kaczor‐Urbanowicz et al., 2017; Musso et al., 2015), and Ebola virus (Vetter et al., 2016). These data demonstrate that saliva is a habitat of pathogenic viruses and suggest it could be a potential source of the spreading of these viruses.
5. PROTECTIVE MEASURES TO MINIMIZE SALIVA‐MEDIATED SPREAD OF DISEASES
Dental procedures can generate saliva‐contaminated splatters, droplets, and aerosols, which can either directly contaminate the exposed skin/eyes/mucosa, or be inhaled by the practitioner, potentially causing cross‐infection. In addition, saliva‐contaminated droplets and aerosols can further contaminate inanimate surfaces in the dental clinical settings, which may also cause nosocomial infection (Peng et al., 2020). Based on our experience on the SARS and COVID‐19 epidemics, some precautions that could reduce the possibility of saliva‐mediated spread of diseases in the dental clinic are suggested as follows.
Patient screening is of the most importance to avoid COVID‐19 transmission in dental clinics. Dental Health Care Personnel should be familiar with COVID‐19 symptoms and able to identify a suspected COVID‐19 patient. A non‐invasive rapid screening test would be of great help to identify positive cases that warrant immediate quarantine or transfer to special clinic for further treatment. Recently, the U.S. Food and Drug Administration (FDA) has authorized an emergency use of a Covid‐19 saliva test (Rutgers Clinical Genomics Laboratory, letter of authorization available at https://www.fda.gov/medical‐devices/emergency‐situations‐medical‐devices/ emergency‐use‐authorizations), which could greatly reduce the risk of occupational infection when performing nasopharyngeal or oropharyngeal collections. Future development of saliva‐based rapid tests on either SARS‐CoV‐2 nucleic acid or antibodies may also have potential to help identify suspected cases in the dental clinic during the epidemic period.
Personal protective equipment is important for infection control in the dental clinic, particularly considering that splatter/droplets contain potential saliva‐borne pathogens. Protective goggles or face shields, masks, gloves, and caps should be regularly worn by the practitioner. When in the epidemic period of contagious diseases such as SARS and COVID‐19, transmission‐based precautions (Figure 1a), including additional protective outwear, surgical mask/N95 respirator and shoe covers, should be adopted by the practitioner to avoid potential droplet/aerosol transmission (Meng et al., 2020; Samaranayake & Peiris, 2004).
FIGURE 1.
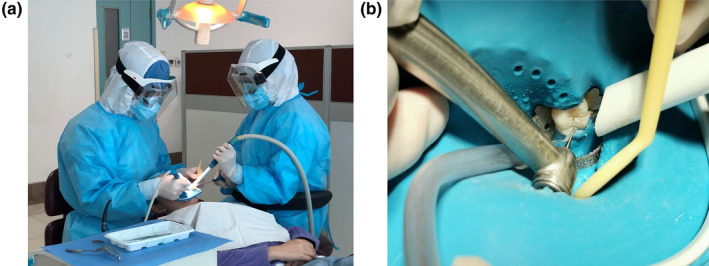
(a) An example of transmission‐based precautions to avoid saliva‐contaminated splatters, droplets and aerosols in the epidemic period of COVID‐19. (b) When rubber dam is applied, extra high‐volume evacuation along with regular saliva ejector should be used to reduce splatters, droplets and aerosols as much as possible
A recent meta‐analysis has shown that a preprocedural mouthrinse can significantly reduce microbial load in dental aerosols (Marui et al., 2019). A preprocedural mouthrinse would be most useful in cases when rubber dam cannot be used. Of note, most of suggestions for preprocedural mouthrinse are based on bacteriology instead of virology. Limited evidence has shown that povidone solutions (0.23%~1%) are effective against SARS‐CoV, after 1‐min in vitro incubation (Kariwa, Fujii, & Takashima, 2006). Hydrogen peroxide (0.5%) has also been shown to inactivate HCoV Strain 229E within 1 min of treatment in vitro (Kampf, Todt, Pfaender, & Steinmann, 2020). However, clinical studies are still needed to evaluate their capability for reducing the salivary load of SARS‐CoV‐2 and saliva‐contaminated droplets/aerosols during dental procedures. When performing intraoral examinations, the use of three‐way syringe should be avoided in order to minimize splatter/droplets during the epidemic period of COVID‐19. Intraoral X‐ray examination will stimulate saliva secretion and coughing, and thus extraoral radiographies, such as panoramic radiography and cone‐beam CT could be alternatives (Meng et al., 2020).
The use of rubber dams can significantly minimize the production of saliva‐contaminated splatters, droplets and aerosols, particularly when high‐speed dental handpieces and ultrasonic devices are used. The application of a rubber dam can significantly reduce airborne particles in an approximately 3‐foot diameter of the operational field by 70% (Samaranayake, Reid, & Evans, 1989). When a rubber dam is applied, extra high‐volume evacuation should be used along with a regular saliva ejector to reduce splatters and droplets (Samaranayake & Peiris, 2004), and four‐hand operation is necessary (Figure 1b). If rubber dam isolation is not possible in some cases, manual devices such as Carisolv and hand scalers are recommended for caries removal and periodontal scaling, in order to minimize saliva contamination.
High‐speed dental handpieces without anti‐retraction valves may aspirate and expel debris and fluids during dental procedures. When a rubber dam is not applied, saliva can be aspirated into the handpiece, and saliva‐borne microbes may further contaminate the dental unit waterlines, thus causing cross‐infection. Our study has shown that the anti‐retraction high‐speed dental handpiece can significantly reduce the backflow of oral microorganisms into the tubes of handpiece and dental unit (Hu, Li, Zuo, & Zhou, 2007). Therefore, the use of dental handpieces without anti‐retraction function should be prohibited during the epidemic period of COVID‐19.
Usually, splatters and droplets can contaminate a 3‐ft diameter range, while aerosols produced can spread further and cause long‐lasting contamination and potential transmission (Zemouri, de Soet, Crielaard, & Laheij, 2017). A recent study published in the New England Journal of Medicine demonstrated that aerosol and fomite transmission of SARS‐CoV‐2 is plausible, since coronavirus remains viable and infectious in aerosols for hours, and on inanimate surfaces up to days (van Doremalen et al., 2020). Aerosol‐generating dental procedures could spread saliva‐contaminated droplets/aerosols to various surfaces and equipment in the dental office, which requires specific disinfection tactics. Good ventilation is critical for the reduction of aerosols in the clinic setting. Strict and regular surface disinfection with alcohol or chlorine disinfectant is also important during the outbreak of COVID‐19. Of note, saliva‐containing waste generated by patients with suspected or confirmed contagious diseases is regarded as infectious medical waste and should be properly disposed accordingly.
6. CONCLUSIONS
Saliva as a body fluid with multiple biological functions which not only protects humans from harmful agents, but also acts as a potential route for the spread of diseases. The pandemic of COVID‐19 should reinforce the awareness of dental professionals on the transmission risk during dental practice. Additional protective measures should be taken at this special moment to minimize potential saliva‐derived hazards to both the dental practitioners and the patients.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. Li, B. Ren, X. Peng, X. Xu, X. Zhou, T. Hu, J. Li, T. Gong, and B. Tang, contributed to conception and design, and drafted and critically revised the manuscript. All authors gave their final approval and are accountable for all aspects of the work.
ACKNOWLEDGEMENTS
This work was supported by Special Funds for Prevention and Control of COVID‐19 of Sichuan University (2020scunCoV‐10008).
Li Y, Ren B, Peng X, et al. Saliva is a non‐negligible factor in the spread of COVID‐19. Mol Oral Microbiol. 2020;35:141–145. 10.1111/omi.12289
Contributor Information
Xin Xu, Email: xin.xu@scu.edu.cn.
Xuedong Zhou, Email: zhouxd@scu.edu.cn.
REFERENCES
- Anschau, V. , & Sanjuan, R. (2020). Fibrinogen gamma chain promotes aggregation of vesicular stomatitis virus in saliva. Viruses, 12(3), 282. 10.3390/v12030282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaseda, A. , Guzmán, M. G. , Hammond, S. , Robleto, G. , Flores, C. , Téllez, Y. , … Harris, E. (2003). Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clinical and Diagnostic Laboratory Immunology, 10(2), 317–322. 10.1128/CDLI.10.2.317-322.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, T. F. , Kournikakis, B. , Bastien, N. , Ho, J. , Kobasa, D. , Stadnyk, L. , … Plummer, F. (2005). Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. Journal of Infectious Diseases, 191(9), 1472–1477. 10.1086/429634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corstjens, P. L. , Abrams, W. R. , & Malamud, D. (2016). Saliva and viral infections. Periodontology 2000, 70(1), 93–110. 10.1111/prd.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Pdel, V. , Gregio, A. M. , Machado, M. A. , de Lima, A. A. , & Azevedo, L. R. (2008). Saliva composition and functions: A comprehensive review. The Journal of Contemporary Dental Practice, 9(3), 72–80. 10.5005/jcdp-9-3-72 [DOI] [PubMed] [Google Scholar]
- Diamond, G. , Beckloff, N. , & Ryan, L. K. (2008). Host defense peptides in the oral cavity and the lung: Similarities and differences. Journal of Dental Research, 87(10), 915–927. 10.1177/154405910808701011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry, J. T. , Birdwell, C. E. , & Scott, R. S. (2018). Epstein‐Barr virus in the pathogenesis of oral cancers. Oral Diseases, 24(4), 497–508. 10.1111/odi.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst, E. J. , & Oppenheim, F. G. (2007). Saliva: A dynamic proteome. Journal of Dental Research, 86(8), 680–693. 10.1177/154405910708600802 [DOI] [PubMed] [Google Scholar]
- Hu, T. , Li, G. , Zuo, Y. L. , & Zhou, X. D. (2007). Risk of hepatitis B virus transmission via dental handpieces and evaluation of an antisuction device for prevention of transmission. Infection Control and Hospital Epidemiology, 28(1), 80–82. 10.1086/510808 [DOI] [PubMed] [Google Scholar]
- Kaczor‐Urbanowicz, K. E. , Martin Carreras‐Presas, C. , Aro, K. , Tu, M. , Garcia‐Godoy, F. , & Wong, D. T. (2017). Saliva diagnostics – Current views and directions. Experimental Biology and Medicine, 242(5), 459–472. 10.1177/1535370216681550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf, G. , Todt, D. , Pfaender, S. , & Steinmann, E. (2020). Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection, 104(3), 246–251. 10.1016/j.jhin.2020.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa, H. , Fujii, N. , & Takashima, I. (2006). Inactivation of SARS coronavirus by means of povidone‐iodine, physical conditions and chemical reagents. Dermatology, 212(Suppl 1), 119–123. 10.1159/000089211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander, P. E. (2011). Multispecies communities: Interspecies interactions influence growth on saliva as sole nutritional source. International Journal of Oral Science, 3(2), 49–54. 10.4248/Ijos11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Guan, X. , Wu, P. , Wang, X. , Zhou, L. , Tong, Y. , … Feng, Z. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. New England Journal of Medicine, 382(13), 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wei, Q. , Alvarez, X. , Wang, H. , Du, Y. , Zhu, H. , … Chen, Z. (2011). Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. Journal of Virology, 85(8), 4025–4030. 10.1128/Jvi.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboobi, N. , Porter, S. R. , Karayiannis, P. , & Alavian, S. M. (2012). Oral fluid and hepatitis A, B and C: A literature review. Journal of Oral Pathology and Medicine, 41(7), 505–516. 10.1111/j.1600-0714.2011.01123.x [DOI] [PubMed] [Google Scholar]
- Malamud, D. , Abrams, W. R. , Barber, C. A. , Weissman, D. , Rehtanz, M. , & Golub, E. (2011). Antiviral activities in human saliva. Advances in Dental Research, 23(1), 34–37. 10.1177/0022034511399282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui, V. C. , Souto, M. L. S. , Rovai, E. S. , Romito, G. A. , Chambrone, L. , & Pannuti, C. M. (2019). Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. Journal of the American Dental Association, 150(12), 1015–1026.e1. 10.1016/j.adaj.2019.06.024 [DOI] [PubMed] [Google Scholar]
- Meng, L. , Hua, F. , & Bian, Z. (2020). Coronavirus disease 2019 (COVID‐19): Emerging and future challenges for dental and oral medicine. Journal of Dental Research, 99(5), 481–487. 10.1177/0022034520914246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso, D. , Roche, C. , Nhan, T. X. , Robin, E. , Teissier, A. , & Cao‐Lormeau, V. M. (2015). Detection of Zika virus in saliva. Journal of Clinical Virology, 68, 53–55. 10.1016/j.jcv.2015.04.021 [DOI] [PubMed] [Google Scholar]
- Musso, D. , Teissier, A. , Rouault, E. , Teururai, S. , de Pina, J. J. , & Nhan, T. X. (2016). Detection of chikungunya virus in saliva and urine. Virology Journal, 13, 102. 10.1186/s12985-016-0556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X. , Xu, X. , Li, Y. , Cheng, L. , Zhou, X. , & Ren, B. (2020). Transmission routes of 2019‐nCoV and controls in dental practice. International Journal of Oral Science, 12(1), 9. 10.1038/s41368-020-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosa, E. J. , Esbenshade, J. C. , Fuller, M. P. , & Weitkamp, J. H. (2012). Cytomegalovirus infection. Pediatrics in Review, 33(4), 156–163. 10.1542/pir.33-4-156 [DOI] [PubMed] [Google Scholar]
- Proctor, D. M. , Fukuyama, J. A. , Loomer, P. M. , Armitage, G. C. , Lee, S. A. , Davis, N. M. , … Relman, D. A. (2018). A spatial gradient of bacterial diversity in the human oral cavity shaped by salivary flow. Nature Communications, 9(1), 681. 10.1038/s41467-018-02900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl, S. (2012). The scientific exploration of saliva in the post‐proteomic era: From database back to basic function. Expert Rev Proteomic, 9(1), 85–96. 10.1586/Epr.11.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, L. P. , & Peiris, M. (2004). Severe acute respiratory syndrome and dentistry – A retrospective view. Journal of the American Dental Association, 135(9), 1292–1302. 10.14219/jada.archive.2004.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, L. P. , Reid, J. , & Evans, D. (1989). The efficacy of rubber dam isolation in reducing atmospheric bacterial‐contamination. ASDC Journal of Dentistry for Children, 56(6), 442–444. 10.1177/00220345890680111601 [DOI] [PubMed] [Google Scholar]
- Seto, W. H. (2015). Airborne transmission and precautions: Facts and myths. Journal of Hospital Infection, 89(4), 225–228. 10.1016/j.jhin.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira, W. L. , Moffa, E. B. , Mussi, M. C. M. , & Machado, M. A. A. M. (2016). Zika virus infection spread through saliva – A truth or myth? Brazilian Oral Research, 30. 10.1590/1807-3107BOR-2016.vol30.0046 [DOI] [PubMed] [Google Scholar]
- Tenovuo, J. (2002). Antimicrobial agents in saliva – Protection for the whole body. Journal of Dental Research, 81(12), 807–809. 10.1177/154405910208101202 [DOI] [PubMed] [Google Scholar]
- To, K.‐W. , Tsang, O.‐Y. , Yip, C.‐Y. , Chan, K.‐H. , Wu, T.‐C. , Chan, J.‐C. , … Yuen, K.‐Y. (2020). Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases. 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan, P. A. , Horby, P. , Dinh, P. N. , Mai, L. T. Q. , Zambon, M. , Shah, J. , … Plant, A. (2007). SARS transmission in Vietnam outside of the health‐care setting. Epidemiology and Infection, 135(3), 392–401. 10.1017/S0950268806006996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , … Munster, V. J. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382(16), 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, P. , Fischer, W. A. , Schibler, M. , Jacobs, M. , Bausch, D. G. , & Kaiser, L. (2016). Ebola virus shedding and transmission: Review of current evidence. Journal of Infectious Diseases, 214(suppl 3), S177–S184. 10.1093/infdis/jiw254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS. Journal of Virology, 94(7), e00127–e220. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W.‐K. , Chen, S.‐Y. , Liu, I.‐J. , Chen, Y.‐C. , Chen, H.‐L. , Yang, C.‐F. , … Chang, S.‐C. (2004). Detection of SARS‐associated coronavirus in throat wash and saliva in early diagnosis. Emerging Infectious Diseases, 10(7), 1213–1219. 10.3201/eid1007.031113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Springer, S. , Mulvey, C. L. , Silliman, N. , Schaefer, J. , Sausen, M. , … Agrawal, N. (2015). Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Science Translational Medicine, 7(293), 293ra104. 10.1126/scitranslmed.aaa8507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, W. J. , Wang, M. L. , Wei, W. , Wang, J. , Zhao, J. J. , Yi, B. , & Li, J. S. (2004). Detection of SARS‐CoV and RNA on aerosol samples from SARS‐patients admitted to hospital. Zhonghua Liu Xing Bing Xue Za Zhi, 25(10), 882–885. 10.1016/j.csr.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , … Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci, 12(1), 8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemouri, C. , de Soet, H. , Crielaard, W. , & Laheij, A. (2017). A scoping review on bio‐aerosols in healthcare and the dental environment. PLoS ONE, 12(5), e0178007. 10.1371/journal.pone.0178007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. Z. , Cheng, X. Q. , Li, J. Y. , Zhang, P. , Yi, P. , Xu, X. , & Zhou, X. D. (2016). Saliva in the diagnosis of diseases. International Journal of Oral Science, 8(3), 133–137. 10.1038/ijos.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


