Abstract
Important health resources are dedicated worldwide to the management of COVID‐19. This new disease, due to its large diffusion, may significantly hamper the prognosis of other pathologies, such as ST‐segment elevation myocardial infarction (STEMI) because of (a) a possible direct negative impact and (b) shortage of first response medical resources and increased delays to reperfusion. We report the case of a 68‐year‐old man admitted for anterior STEMI and asymptomatic COVID‐19. Due to extended transportation delays to a cathlab, he received intravenous fibrinolytic therapy, which failed. Reperfusion was achieved with rescue coronary angioplasty, but the patient experienced two episodes of acute stent thrombosis at 2‐ and 36‐hr following admission and despite optimal medical therapy. He finally died because of cardiogenic shock. This raises concerns about a possible increase in platelet aggregability associated with COVID‐19 leading to an increased risk of stent thrombosis, particularly in the context of STEMI. This pleads for the promotion of primary coronary angioplasty as the first‐choice revascularization technique in this population and the use of new generation P2Y12 inhibitors. In addition, the use of GPIIb/IIIa inhibitors may be considered in every STEMI patient with COVID‐19 to prevent the risk of acute stent thrombosis.
Keywords: acute myocardial infarction, antithrombotic treatment, viral infection
1. INTRODUCTION
COVID‐19 spreads rapidly worldwide and has disastrous consequences in most countries. But while more and more medical resources are dedicated to the management of COVID‐19 patients, intensive cardiac care units still receive patients with acute coronary syndromes (ACS).
Recent publications reported that there were evidence of myocardial injury associated with SARS‐CoV‐2 infection, associated with increased mortality, independently of others risk factors of COVID‐19's negative outcomes. 1 Yet, COVID‐19 is expected to have a direct negative impact in ST‐segment elevation myocardial infarction (STEMI) patients, with more subjects experiencing acute heart failure. 2 Its association with the containment measures may further worsen the prognosis of these patients due to an increase in the delay from the onset of symptoms to first‐medical contact (FMC), the shortage of first response medical resources and increased delays from FMC to primary percutaneous coronary intervention (PCI) as most emergency medical transport resources are dedicated to COVID‐19 patients' management. This was confirmed by Tam et al. who reported longer delays from the onset of symptoms to FMC (318 vs. 82.5 min), door to device (110 vs. 84.5 min), and cathlab arrival to device (33 vs. 20.5 min) compared to pre‐pandemic activity. 3 Due to these increased delays, use of intravenous fibrinolytic therapy in STEMI is more often encouraged. 4 , 5
But regarding the widespread of COVID‐19 and the fact that many patients may be asymptomatic, this strategy may expose to significant worst outcomes in patients combining STEMI and COVID‐19.
We report here the case of a patient admitted for acute anterior STEMI and who was secondarily diagnosed with COVID‐19.
2. CASE REPORT
On March 31, a 68‐year‐old diabetic male presented with a 4 hr acute chest pain lasting in a non‐cathlab equipped hospital. He was diagnosed with anterior STEMI and immediately received dual antiplatelet therapy (DAPT) combining ticagrelor 180 mg and aspirin 250 mg linked with a bolus of intravenous unfractionated heparin. Because of an anticipated extended delay to primary PCI due to the mobilization of all available transportation resources for COVID‐19 patients, intravenous fibrinolytic therapy with tenecteplase was proposed. As recommended, after the onset of thrombolysis, emergency transportation to a cathlab‐equipped hospital was performed. Upon admission in the cathlab, rescue PCI of the proximal left anterior descending artery (LAD) with stent implantation was performed due to persistent coronary occlusion. Two hours later, the patient presented recurrent chest pain, nonsustained ventricular tachycardia and cardiogenic shock. A new emergency coronary angiography revealed acute LAD stent thrombosis that was treated with catheter thrombectomy and balloon angioplasty. DAPT was modified, replacing ticagrelor by prasugrel (with 60 mg loading dose). Left ventricle ejection fraction was estimated at 15%. Inotropic support combining dobutamine infusion and intra‐aortic balloon pump was started as well as therapeutic anticoagulation with intravenous unfractionated heparin.
Because of an early home self‐controlled low body temperature at 34°C (94 °F), SARS‐CoV‐2 PCR from nose swab was performed and confirmed COVID‐19 infection. Of note, at admission, body temperature had normalized, no other sign of infection was noted, and biology only revealed mild leucocytes elevation (14.3 G/L, N < 10), mild C‐reactive protein elevation (33.5 mg/L, N < 5) and moderate fibrinogen elevation (5.75 g/L, N < 4).
Thirty‐six hours later, while the patient was still free from COVID‐19 symptoms, he presented a new episode of chest pain with reappearance of ST‐segment elevation on ECG despite DAPT and therapeutic intravenous unfractionated heparin therapy. Emergency coronary angiography revealed a recurrence of extensive LAD stent thrombosis (see Figure 1). Catheter thrombectomy confirmed the massive thrombotic burden but failed to provide efficient reperfusion due to refractory no reflow. The patient finally died 24 hr later from hemodynamic deterioration.
FIGURE 1.
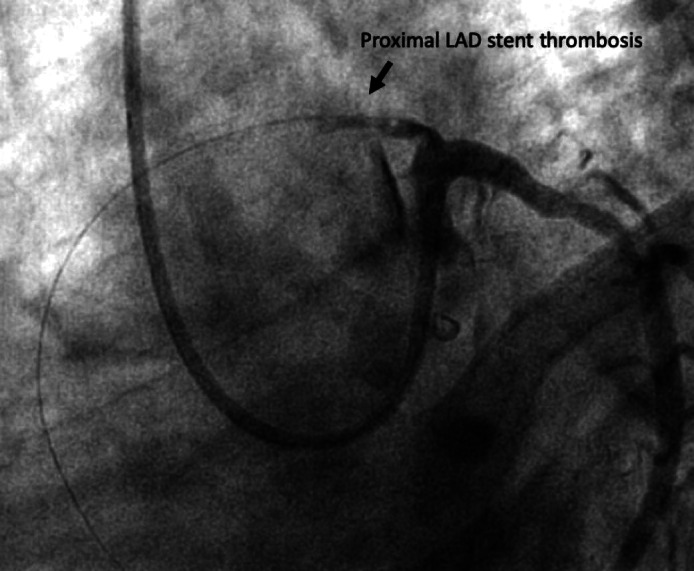
Coronary angiography image showing extensive proximal left anterior descending coronary artery stent thrombosis (caudal left‐anterior‐oblique view)
3. DISCUSSION
Beside the post‐fibrinolysis coagulation rebound that may induce acute coronary reocclusion, 6 we hypothesized that SARS‐CoV‐2 infection may have played a role in this unfavorable evolution. COVID‐19 has significant impacts on patients coagulation. 7 Yet, there is to date no description of COVID‐19 impact on platelet function apart from the sepsis‐associated intravascular disseminated coagulopathy with thrombocytopenia in case of severe infection. 8 In this case combining asymptomatic COVID‐19 and STEMI, despite up‐to‐date optimal antithrombotic medical therapy, there seems to be an increase in the risk of stent thrombosis due to a possible excessive platelet aggregability. Considering the incidence of COVID‐19 that is raising worldwide and the high prevalence of coronary artery disease, we may be exposed to a significant number of cases of patients experiencing STEMI and COVID‐19 at the same time. We may even expect an increase in the incidence of STEMI as it is well accepted that acute viral infection increases the risk of acute myocardial infarction, mainly through acute inflammation and endothelial dysfunction. 9 , 10 Thus, this case and epidemiologic evidences raise concerns about a strategy that would favor intravenous fibrinolytic therapy over timely primary PCI as: (a) COVID‐19 may reduce the efficacy of fibrinolytic therapy, (b) the combination of COVID‐19 and fibrinolytic therapy may further increase the risk of subsequent stent thrombosis.
We propose that (a) timely primary PCI remains the first choice over fibrinolytic therapy in STEMI patients, which means that primary care organization must still prioritize emergency transportation of STEMI patients to cathlabs in order to avoid extensive myocardial necrosis, (b) physicians in charge should prefer new generation P2Y12 inhibitors to clopidogrel, according to ACC/AHA and ESC guidelines, and (c) post‐primary PCI and stent implantation GPIIb/IIIa inhibitors therapy may be considered in every patient with STEMI and suspected COVID‐19 infection, even without high thrombotic burden, to prevent the risk of acute stent thrombosis.
Of course, we need data from large registries to confirm this suspected increased risk of stent thrombosis in these patients combining STEMI and COVID‐19 and this report aims mainly to draw attention toward this particular situation that may become more and more frequent in our cardiology units.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Fabrice Ivanes designed the work. Carl Semaan, Thibaud Genet, and Fabrice Ivanes analyzed and interpreted the data. Thibaud Lacour and Fabrice Ivanes wrote the manuscript. All the authors approved the final version.
ACKNOWLEDGMENTS
We would like to acknowledge Dr Martine Ferrandière and Prof Denis Angoulvant for their assistance in the management of the patient.
Lacour T, Semaan C, Genet T, Ivanes F. Insights for increased risk of failed fibrinolytic therapy and stent thrombosis associated with COVID‐19 in ST‐segment elevation myocardial infarction patients. Catheter Cardiovasc Interv. 2021;97:E241–E243. 10.1002/ccd.28948
REFERENCES
- 1. Bonow RO, Fonarow GC, O'Gara PT, et al. Association of Coronavirus Disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.1105. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, et al. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. http://www.nature.com/articles/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tam C‐CF, Cheung K‐S, Lam S, et al. Impact of Coronavirus Disease 2019 (COVID‐19) outbreak on ST‐segment‐elevation myocardial infarction Care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from ACC's interventional council and SCAI. J Am Coll Cardiol. 2020. 10.1016/j.jacc.2020.03.021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID‐19: the protocols from Sichuan Provincial People's Hospital. Intensive Care Med. 2020. 10.1007/s00134-020-05993-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moser M, Nordt T, Peter K, et al. Platelet function during and after thrombolytic therapy for acute myocardial infarction with reteplase, alteplase, or streptokinase. Circulation. 1999;100:1858‐1864. [DOI] [PubMed] [Google Scholar]
- 7. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0188. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. 2020. 10.1111/jth.14817. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225:293‐296. [DOI] [PubMed] [Google Scholar]
- 10. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611‐2618. [DOI] [PubMed] [Google Scholar]


