Acute promyelocytic leukaemia and COVID‐19 are two conditions associated with severe coagulopathy. We present here the multiple haemostasis abnormalities observed in a patient with concomitant acute promyelocytic leukaemia and COVID‐19. The clinical consequences were dramatic and led to the death of the patient.
A 62‐year‐old woman was admitted on April 13, 2020 for unexplained asthenia, dyspnoea, and uncontrolled epistaxis. Clinical examination revealed pallor, left periorbital ecchymosis due to a recent fall, intrabuccal haemorrhagic bullae, persistent epistaxis, and mild splenomegaly. Oxygen saturation was 96%. Chest computed tomography (CT) was consistent with moderate COVID‐19 pneumonia (nine points on a 0‐25 scale). 1 The quantitative reverse transcription polymerase chain reaction (qRT‐PCR) of a nasopharyngeal swab was positive for SARS‐CoV‐2.
Blood cell count denoted severe anaemia (haemoglobin: 6·8 g/dl) and thrombocytopenia (13 × 109/l) as well as hyperleucocytosis (20·7 × 109/l). Differential count found mild neutropenia (1·45 × 109/l), normal lymphocyte count (2·28 × 109/l) and blast cells (14·7 × 109/l). The patient was referred to our centre on the evening of April 15. Bone marrow was infiltrated by 76% of CD34+, CD33+ and CD117+ microgranular blasts with rare Auer rods. Karyotype identified a t(15;17)(q24;q21) translocation and a PML‐RARα fusion gene was confirmed by fluorescence in situ hybridization (FISH). These findings led to a diagnosis of microgranular variant of acute promyelocytic leukaemia (AML M3v).
Biological inflammatory syndrome was moderate (C‐reactive protein: 9·2 mg/l, normal value: <4; procalcitonin 0·63 µg/l, normal value < 0·05). Haemostasis analysis showed diffuse intravascular coagulation (DIC) signs: increased prothrombin time (22·2 s for a laboratory reference of 13·2), mild decrease of fibrinogen level (1·93 g/l, normal value 2–4) and major increase in D‐dimers (>20 µg/ml, normal value < 0·5). Activated partial thromboplastin time ratio was shortened (0·80). ISTH (International Society of Thrombosis and Haemostasis) DIC score was positive (7 points). High levels of factor VIII (599%, normal value: 60–150) and von Willebrand factor antigen (602%, normal value: 50–150) were also observed. Antithrombin III level was normal (103%, normal value >80). Lupus anticoagulant testing was negative.
In the morning of April 16, we initiated hydroxycarbamide and all‐trans retinoic acid and the patient received red blood cell and platelet transfusions. In the evening, the patient suddenly developed right hemiplegia. There was no worsening of DIC. Computed tomography (CT) angiography showed left middle cerebral artery occlusion (Fig 1). The non‐contrast CT ASPECTS (Alberta Stroke Program Early CT) score was 9. Endovascular thrombectomy was successfully performed under general anaesthesia with a complete vessel recanalization. According to standard practice, it was decided to avoid anticoagulants and antiplatelets for 24 h. SARS‐CoV‐2 qRT‐PCR performed on the thrombus was negative. Nine hours after thrombectomy the clinical picture worsened with fixed bilateral mydriasis. Repeat CT imaging did not show haemorrhagic transformation; surprisingly there were multiple new arterial occlusions: left internal carotid and middle cerebral arteries, right internal carotid artery, basilar trunk and left posterior cerebral artery. A second thrombectomy was not performed. The patient died two days later.
Fig 1.
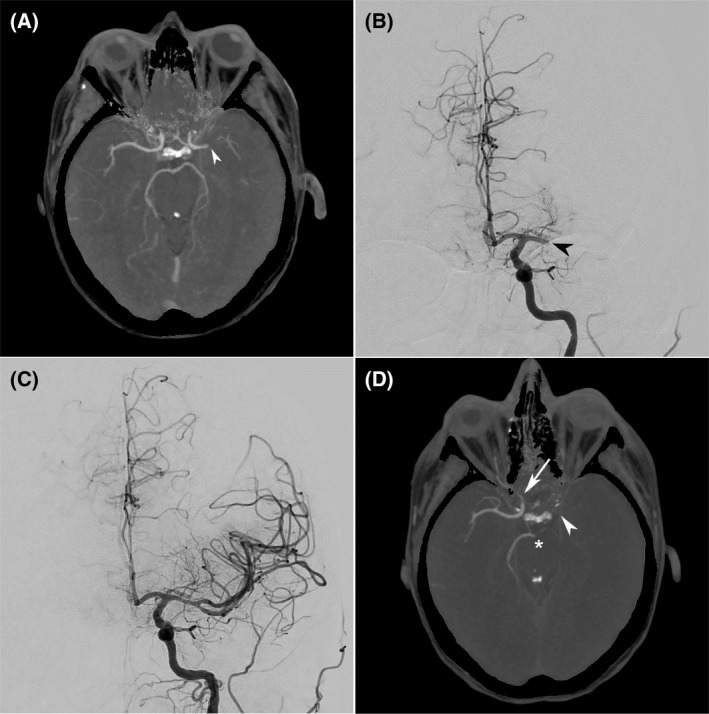
(A) Initial CT angiography 1·5 hours after onset of right hemiplegia, demonstrating left middle cerebral artery occlusion (arrowhead). Angiography before (B) and after (C) endovascular thrombectomy was performed with complete recanalization 3 h and 15 min after symptom onset. (D) Nine hours after thrombectomy, the patient failed to regain consciousness and had bilateral fixed mydriasis. CT angiography demonstrated multiple new arterial occlusions: left internal carotid and middle cerebral arteries (arrowhead), right cervical and cavernous internal carotid artery (arrow), and distal basilar trunk and left posterior cerebral artery (asterisk).
Most promyelocytic leukaemias present with coagulopathy combining DIC, thrombocytopenia and hyperfibrinolysis. 2 Biological manifestations include an increase in activated partial thromboplastin time and prothrombin time, severe thrombocytopenia, decrease in fibrinogen and elevation in D‐dimers. In contrast with DIC from other causes, levels of protein C, protein S and antithrombin III are usually not reduced. Coagulopathy usually worsens when antileukaemic treatment is initiated. While haemorrhages are seen in most patients, thrombosis is less frequent and has been reported in approximately 10% of cases. Most thrombotic events occur in small vessels.
COVID‐19 is also associated with thrombotic events. 3 , 4 The main explanations for these thrombotic events are inflammatory syndrome, hypoxia, and coagulopathy. Biological abnormalities include increase in D‐dimers, fibrinogen, factor VIII and von Willebrand factor, possible mild thrombocytopenia and limited and variable effects on prothrombin time and activated partial thromboplastin time. 4 Presence of lupus anticoagulant is frequent in severe COVID‐19. Microvascular system damages caused by inflammation are an additional factor of thrombosis in small vessels. 5 Venous thromboses are more frequent than arterial occlusions. 5
Recurrent and extensive thrombosis of cerebral arteries was concomitant with haemorrhages as a result of leukaemia‐related DIC, COVID‐related coagulopathy and severe thrombocytopenia. The role of heparin is controversial in promyelocytic leukaemia. Anticoagulation is recommended in severe COVID‐19 but often fails to prevent thrombosis when given at prophylactic or standard curative doses. 6 , 7 , 8 The haemorrhagic syndrome, the very low platelet count and the recent cerebral thrombectomy represented contraindications to anticoagulation.
Conflicts of interest
All authors declare no conflict of interest in relation with the submitted work.
Author contributions
LM, RB and RH designed the study and analysed the data. MB, RP, LS, KB; JG, FS and CS contributed in acquisition and interpretation of the data. MB, RP, LS and RH wrote the paper. LM, RB, FS, RP, KB, JG and CS critically reviewed the manuscript. All authors approved the final version.
References
- 1. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology. 2020;200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David S, Mathews V. Mechanisms and management of coagulopathy in acute promyelocytic leukemia. Thromb Res. 2018;164(Suppl 1):S82–S88. [DOI] [PubMed] [Google Scholar]
- 3. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin‐Loeches I, Browne P, et al. COVID‐19 coagulopathy in Caucasian patients. Br J Haematol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients in severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID‐19 patients: emerging evidence and call for action. Br J Haematol; 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


