To the Editor,
The World Health Organization declared the outbreak of novel coronavirus disease 2019 (COVID‐19) a Public Health Emergency of International on 30 January 2020. 1 It is well documented that certain viral infections, such as measles, have been known to aggravate pulmonary tuberculosis (PTB), presumably as a result of depressed cellular immunity. 2 , 3 Despite their vulnerability as a population, to date, most studies have focused on COVID‐19 infection in patients without current respiratory disease. The clinical features and treatment of tuberculosis (TB) patients with COVID‐19 are unclear and understudied. To the best of our knowledge, the coinfection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and Mycobacterium TB has not been reported previously. Therefore, we provide information and experience with the treatment of TB cases with confirmed COVID‐19 infection.
Data were derived from 139 confirmed cases, which were hospitalized in Wenzhou Sixth People's Hospital from 17 January to 6 March 2020. Three TB cases with COVID‐19 infection were confirmed by a real‐time fluorescence polymerase chain reaction assay. All three patients were males, ages 26 to 76 years, and were prospectively followed from hospital admission to discharge.
Table 1 illustrates the clinical features, laboratory tests, clinical prognosis, treatments, and outcomes of the three patients. According to this epidemiological investigation, all patients were in close contact and infected from others with confirmed infections of COVID‐19. Patients 1 and 2 had PTB history and were administered quadruple antituberculosis therapy, while patient 3 had untreated TB for 50 years. The cavitating lesion of patient 3 in the right upper lobe remained unchanged for many years. Considering the harmfulness of the SARS‐CoV‐2 specimen, the hospital restricted the examination of sputum Xpert, T‐SPOT.TB, the culture of Mycobacterium TB, and so forth. The onset of their symptoms varied from a dry cough to chest tightness to persistent fever. Other symptoms included chest pain, diarrhea, and dyspnea. Patients 1 and 3, who were of older age, had hypoxemia and developed a critical type and severe type of COVID‐19, respectively. Laboratory tests showed all of them had low lymphocyte count and increased levels of C‐reactive protein, lactic dehydrogenase, and erythrocyte sedimentation rate. Computed tomography (CT) scans showed all of the cases presented with multiple bilateral ground‐glass opacities and consolidation, with air bronchogram.
Table 1.
Clinical features, laboratory tests, clinical prognosis, treatments, and outcomes of the three patients
| Patient number | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age, y | 67 | 26 | 76 |
| Sex (M/F) | M | M | M |
| BMI | 24.2 | 28.73 | 17.96 |
| Past history | TB (treated 6 y ago) | TB (treated 2 y ago) | TB (for 50 y, nontreated) |
| HBP | |||
| Exfoliative dermatitis | |||
| Arthrolithiasis | |||
| Smoking history | + | − | + |
| Imported case from Wuhan | + | + | − |
| Clinical features | |||
| Fever (T max,℃) | 38.9 | 38.2 | 38.9 |
| Dry cough | + | + | + |
| Chest tightness | + | − | + |
| Chest pain | − | − | + |
| Dyspnea | + | − | + |
| Diarrhea | − | + | − |
| Family cluster | + | + | + |
| Oxygen saturation on room air (%) | 89↓ | 97 | 96 |
| Respiratory rate | 22 | 19 | 20 |
| Laboratory findings | |||
| Lowest leukocyte count (×109/L) | 3.3↓ | 3.2↓ | 3.9↓ |
| Lowest lymphocyte count (×109/L) | 0.5↓ | 0.8↓ | 0.3↓ |
| Lowest PaO2, kPa | 6.7↓ (FiO2 33%) | NA | 9.3↓ (FiO2 33%) |
| Highest serum ALT, U/L | 39 | 33 | 22 |
| Lowest serum ALB, g/L | 32.5↓ | 43.9 | 31.6↓ |
| Highest serum CKP, U/L | 142 | 41.1 | 105 |
| Highest serum LDH, U/L | 396↑ | 372↑ | 328↑ |
| Highest CRP, mg/L | 124.2↑ | 29.2↑ | 77.1↑ |
| Highest ESR, mm/h | 91↑ | NA | 38↑ |
| Radiological findings of the thorax (radiograph/computed tomography) | |||
| Initial change | Multiple bilateral mass ground‐glass opacities, stripe of high‐density shadow on right upper lobe (day 7) | Multiple ground‐glass opacities on lower lobe bilaterally, with mediastinal lymphadenopathy (day 9) | Pathy ground‐glass opacities on left lower lobe, stripe of high‐density shadow on right upper lobe with a cavitating lesion in the upper lobe of the right lung (day 2) |
| Progressive change | Increased bilateral multiple ground‐glass opacities and consolidation (day 13) | Absorption of the ground‐glass opacities (day 20) | Increased multiple ground‐glass opacities and consolidation on lower lobe bilaterally (day 5) |
| Absorption of some area (day 43) | |||
| Absorption of some area (day 18) | |||
| Absorption of some area (day 10) | |||
| Increased consolidation (day 15) | |||
| Absorption than day 18 | |||
| Increased consolidation (day 22) | |||
| (day 24) | |||
| Absorption of some area (day 28) | |||
| Unchanged image (day 35) | |||
| Absorption of some area (day 40) | |||
| Comorbid conditions | |||
| Hyoxemia | + | − | + |
| Glucose level abnormal | + | − | + |
| ARDS | + | − | − |
| Bacterial infection | + | + | + |
| Medicine rash | − | + | − |
| Treatment and outcome | |||
| Aerosol therapy with interferon alfa‐2b | + | + | + |
| Antivirus medicine | Lopinavir + Ritonavir | Lopinavir + Ritonavir | Lopinavir + Ritonavir |
| Arbidol | Arbidol | Arbidol | |
| Methylprednisolone | + (day 10‐11) | − | + (day 5‐12) |
| Antibiotics | + | + | + |
| Antituberculosis | − | − | + |
| Probiotics | + | + | + |
| Traditional Chinese medicine | + | + | + |
| Intravenous immunoglobulin | + | − | + |
| Ventilatory support | Noninvasive ventilation | − | Nasal cannula |
| Maximum oxygen requirement (FiO2) | 50% | − | 41% |
| Outcome of symptoms | Relief | Relief | Relief |
| Last OI 325 | Last OI 396 | ||
| Hospital stay, d | 37 | 28 | 24 |
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; BMI, body mass index; CKP, creatine kinase; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HBP, high blood pressure; LDH, lactic dehydrogenase; NA, not applicable; OI, Oxygenation Index = PaO2/FiO2; TB, tuberculosis.
All the patients had antivirus therapy including lopinavir, ritonavir, and arbidol with a long prognosis. Patient 1 progressed to respiratory failure type 1 and acute respiratory distress syndrome (ARDS) on day 10 after onset. He had treatment of noninvasive ventilation support. Both patients 1 and 3 were severe cases and had treatment of methylprednisolone. Since patient 3 had untreated TB for many years, he received antituberculosis therapy combined with glucocorticoid therapy. Cases 1 and 3 both had glucose level abnormalities and bacterial infections. They were put on antibiotic therapy and the symptoms relieved later. Patient 2 developed skin rashes with pruritus all over his body, which is considered an adverse reaction of the antiviral treatment. His symptoms improved after he stopped the antivirus therapy. All of the patients recovered and were discharged from the hospital. However, on a ninth day after discharge, patient 2 had a positive recurrence of SARS‐CoV‐2 RNA and returned to the hospital to remain in isolation and under observation.
Among these three COVID‐19 combined patients with TB, two who were older in age developed severe cases of COVID‐19 and one which was ARDS. All had a long recovery and it was difficult to resolve the problem of low Oxygenation Index. The CT images showed the COVID‐19 lesions and TB lesions coexist and performed differently (Figure 1 and Figure S1). The COVID‐19 lesions were mainly performed as ground‐glass lesions and consolidation, mainly located in the peripheral zone. Mycobacterium TB lesions were mainly performed as a stripe of high‐density shadow with a cavitating lesion, mainly located in the upper lung. Limited by the small sample size, we cannot draw the conclusion. Future large sample‐sized study address on the pathology of COVID with CT imaging are warranted.
Figure 1.
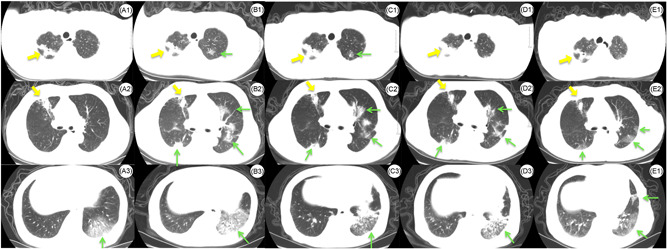
Axial CT images of the chest in patient 3 with the COVID‐19 and tuberculosis coinfection. A, CT obtained on day 2. A1, Stripe of high‐density shadow with a cavitating lesion in the right upper lobe. A2, Stripe of high‐density shadow in the right upper lobe. A3, Pathy ground‐glass opacities (GGOs) in the left lower lobe. B, CT obtained on day 7. B1, Stripe of high‐density shadow with a cavitating lesion in the right upper lobe, slight GGO in the left upper lobe. B2, Stripe of high‐density shadow on the right middle lobe, subpleural (GGOs) in the right upper lobe. Multifocal, limited GGO is seen in the left lungs. B3, Increased multiple GGOs and consolidation in the left lower lobe with air bronchograms. C, CT obtained on day 16. C1, Stripe of high‐density shadow with a cavitating lesion in the right upper lobe, slight GGO in the left upper lobe. C2, Stripe of high‐density shadow on the right middle lobe, absorption of GGO bilaterally. C3, GGO and consolidation in left lower lobe. D, CT obtained on day 22. D1, Stripe of high‐density shadow with a cavitating lesion in the right upper lobe, absorption of GGO in the left upper lobe. D2, Stripe of high‐density shadow on the right middle lobe, absorption of GGO bilaterally. D3, GGO and consolidation in left lower lobe. E, Follow‐up CT obtained on day 59. E1, Stripe of high‐density shadow with a cavitating lesion in the right upper lobe. E2, Stripe of high‐density shadow on right middle lobe, absorption of GGO bilaterally. E3, Absorption of GGO in the left lower lobe. Yellow arrows demonstrated the lesion of tuberculosis. Green arrows demonstrated the lesion of COVID‐19. COVID‐19, coronavirus disease 2019; CT, computed tomography
TB is the type of infection that requires cellular immunity, and nearly 100 years ago lung function studies with TB showed evidence of restrictive lung disease. In patients infected with Mycobacterium tuberculosis, whether treated or untreated, a variety of pulmonary and extrapulmonary sequelae and complications can occur, which include bronchiectasis, tracheobronchial stenosis, and broncholithiasis. 4 Structural changes lead to obstructive, restrictive, or mixed patterns of impaired pulmonary function. Studies in patients with PTB have demonstrated that 33.3% to 94.0% of such patients develop the impaired pulmonary function. 5 The risk factors for TB patients with reduced pulmonary function are having previously had culture‐positive PTB, being over 50 years of age, having a low level of education, and having experienced recurrence of TB. 6 This study suggests that previous lung disease such as treated or untreated mycobacterium TB and old age are independent risk factors of a worse prognosis of those infected with COVID‐19. Using a glucocorticoid for a short period of time in the early stages of prognosis could reduce the inflammation, but longer term usage could result in the risk of bacterial infections and abnormal glucose levels. These case reports remind us of the importance of strict isolation of patients with COVID‐19, careful use of steroids for their case management, and the possibility of coinfection with TB in COVID‐19 patients with incomplete recovery.
The presented case series demonstrates PTB patients with COVID‐19. In this study, elderly patients with TB easily progressed to the severe type of COVID‐19 and had a long recovery process. These case reports remind us of the possibility of coinfection with TB in COVID‐19 patients with incomplete recovery, as well as the importance of careful use of steroids for their case management.
FUNDING INFORMATION
This study was supported by the Major Project of Wenzhou Municipal Science and Technology Bureau (ZY202004).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
GH and JC conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. WS conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. JS and JD collected data, and reviewed and revised the manuscript. JW carried out the analyses, critically reviewed the manuscript for important intellectual content, and reviewed and revised the manuscript. XJ and MG carried out the analyses, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ETHICS STATEMENT
This study was approved with the written consent by the Ethics Committee of Wenzhou Central Hospital (No. L2020‐02‐004X).
Supporting information
Supporting information
Supporting information
Funding Information Major Project of Wenzhou Municipal Science and Technology Bureau, Grant/Award Number: ZY202004
Guiqing He, Jing Wu, Jichan Shi, and Jianyi Dai contributed equally to this study.
Contributor Information
Xiangao Jiang, Email: xiangao368@163.com.
Wenjie Sun, Email: wsun@fiu.edu.
Jing Cai, Email: michellecai666@hotmail.com.
REFERENCES
- 1. World Health Organization . Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Accessed 15 February 2020.
- 2. Griffin DE, Bellini WJ. Measles virus. In: Fields BN, Knipe DM, Howley PM, eds. Virology. Philadelphia, PA: Lippincott‐Raven; 1996:1267‐1312. [Google Scholar]
- 3. Kempe CH, Fulginiti VA. The pathogenesis of measles virus infection. Arch Gesamte Virusforsch. 1965;16:103‐128. [DOI] [PubMed] [Google Scholar]
- 4. Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21(4):839‐858. discussion 859‐860. [DOI] [PubMed] [Google Scholar]
- 5. Stepanian I. Bronchial impotence in patients with pulmonary tuberculosis [article in Russian]. Tuberk Biolezni Legk. 2013;4(1):6‐11. [Google Scholar]
- 6. Chushkin MI, Ots ON. Impaired pulmonary function after treatment for tuberculosis: the end of the disease? J Bras Pneumol. 2017;43(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


