Abstract
BACKGROUND/OBJECTIVES
Previous studies have reported that older patients may experience worse outcome(s) after infection with severe acute respiratory syndrome coronavirus‐2 than younger individuals. This study aimed to identify potential risk factors for mortality in older patients with coronavirus disease 2019 (COVID‐19) on admission, which may help identify those with poor prognosis at an early stage.
DESIGN
Retrospective case‐control.
SETTING
Fever ward of Sino‐French New City Branch of Tongji Hospital, Wuhan, China.
PARTICIPANTS
Patients aged 60 years or older with COVID‐19 (n = 244) were included, of whom 123 were discharged and 121 died in hospital.
MEASUREMENTS
Data retrieved from electronic medical records regarding symptoms, signs, and laboratory findings on admission, and final outcomes of all older patients with COVID‐19, were retrospectively reviewed. Univariate and multivariate logistic regression analyses were used to explore risk factors for death.
RESULTS
Univariate analysis revealed that several clinical characteristics and laboratory variables were significantly different (ie, P < .05) between discharged and deceased patients. Multivariable logistic regression analysis revealed that lymphocyte (LYM) count (odds ratio [OR] = 0.009; 95% confidence interval [CI] = 0.001‐0.138; P = .001) and older age (OR = 1.122; 95% CI = 1.007‐1.249; P = .037) were independently associated with hospital mortality. White blood cell count was also an important risk factor (P = .052). The area under the receiver operating characteristic curve in the logistic regression model was 0.913. Risk factors for in‐hospital death were similar between older men and women.
CONCLUSION
Older age and lower LYM count on admission were associated with death in hospitalized COVID‐19 patients. Stringent monitoring and early intervention are needed to reduce mortality in these patients. J Am Geriatr Soc 68:E19–E23, 2020.
Keywords: COVID‐19, mortalityolder patientsrisk factors
INTRODUCTION
In December 2019, a cluster of cases of novel virus‐related pneumonia occurred in Wuhan, China, 1 and the number of infected persons has increased rapidly due to the high transmissibility and infectivity of the causative virus. 2 The virus has been isolated and identified as a novel enveloped RNA beta‐coronavirus (termed severe acute respiratory syndrome coronavirus‐2 [SARS CoV‐2]) using epidemiological, clinical, laboratory, and genetic methods. 3 The World Health Organization has named this novel pneumonia as coronavirus disease 2019 (COVID‐19) and declared it a global pandemic. 4
The city of Wuhan—the epidemic source of COVID‐19—medical institutions and clinicians have been facing unprecedented challenges due to the rapid spread of the COVID‐19 and increase in the number of patients. As of March 5, 2020, a total of 49,797 cases were confirmed in Wuhan, of which 2,328 died of COVID‐19. 5 The Sino‐French New City Branch of Tongji Hospital was designated by the Chinese government as a specified hospital for treating severely and critically ill patients with COVID‐19.
Older patients with COVID‐19 have been reported to exhibit relatively higher mortality and severity of illness than younger patients.6, 7 However, to the best of our knowledge, no previous study has reported definitive outcomes in older patients. Accordingly, this retrospective, single‐center study aimed to identify early‐stage risk factors associated with death, and to compare the clinical characteristics on admission of in‐hospital deceased and discharged older patients with COVID‐19.
METHODS
Participants
Participants were identified from inpatients of the Sino‐French New City Branch of Tongji Hospital, a specialized hospital with 23 critical illness wards (including two intensive care units) with 1,085 beds for treating COVID‐19, by searching the hospitalʼs electronic medical records between January 29 and March 5, 2020. Patients aged 60 years or older, who were diagnosed with COVID‐19 with definite outcomes (discharged or died in hospital) by March 5, 2020, were enrolled. The diagnosis of all included patients was confirmed according to the Diagnosis and Treatment Guideline for COVID‐19 published by the National Health Commission of Peopleʼs Republic of China. 5 Patients who died of COVID‐19 and related complications in the hospital were enrolled as the deceased group, while those who fulfilled the criteria for cure of COVID‐19, as per the guideline, 5 and were discharged from hospital were enrolled as the discharged group. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Data Collection
Demographic information and data regarding symptoms, signs, and laboratory findings on admission and outcomes were retrieved from electronic medical records and retrospectively reviewed and analyzed. At least two experienced researchers were responsible for data collection, and a trained team of physicians reviewed and analyzed data from each patient. The date of disease onset was defined as the day of the first self‐reported onset of symptoms. Peripheral oxygen saturation (SpO2) was measured in the patientʼs oxygen‐absorbing state because all patients were severely ill on admission or transferred from the fever clinic or other medical institutions with oxygen devices.
Statistical Analysis
Data are expressed as median (interquartile range) for continuous variables and counts and frequency (number [percentage]) for categorical variables. Comparison of categorical variables between the discharged and deceased groups was performed using Pearsonʼs χ2 test with continuity correction or Fisherʼs exact test, where appropriate. The Mann‐Whitney U test was used to compare differences in continuous variables between the two groups because they were all nonnormally distributed. Multivariable logistic regression modeling and receiver operating characteristic (ROC) curves were used to explore independent risk factors for death. Continuous variables were treated as continuous measures in the model. The same univariate statistical methods were applied to compare differences between male and female patients, and to explore risk factors for mortality stratified according to sex. Statistical analyses were performed using SPSS version 21.0 (IBM Corporation). Differences were considered to be statistically significant when two‐sided P values were < .05.
RESULTS
A total of 244 older patients with a definite clinical outcome recorded by March 5, 2020, were enrolled in the study, including 123 who were discharged and 121 who died (Table 1). The median age of the discharged patients was 67 years, while that of the deceased group was 72 years (P < .001). Among all patients, 58.5% in the discharged group and 32.2% in the deceased group were female (P < .001). Most patients exhibited fever (86.5%) and respiratory symptoms (88.5%), and the main respiratory symptom was dry cough (73.4%). Approximately one‐third of patients experienced digestive symptoms, such as diarrhea. Among all patients, 21% had diabetes, while 14.4% had coronary heart disease. These two common chronic comorbidities of older patients were not associated with the outcome, while the proportion of hypertension was significantly different between the two groups (P = .042). While 16.7% of deceased patients had a history of respiratory problems, only 3.3% of the discharged patients had a history of respiratory problems (P < .001). Approximately 13.1% of the patients were admitted with consciousness disorders. Approximately one‐third of the patients were admitted with oxygen saturation less than 90%. The median SpO2 was 97% and 90% for discharged and deceased patients, respectively (P < .001). The respiratory rate and heart rate of the deceased patients were higher than values of those who were discharged (P < .001). Vital signs on admission, including consciousness disorders, SpO2 less than 90%, respiratory rate greater than 20 breaths/min, and heart rate greater than 100 beats/min, were often associated with poor outcomes.
Table 1.
Demographic Information, Clinical Characteristics, and Laboratory Findings on Admission
| Characteristic | Discharged (n = 123) a | Deceased (n = 121) a | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | <.001 | ||
| Male | 51 (41.5) | 82 (67.8) | |
| Female | 72 (58.5) | 39 (32.2) | |
| Age, y | 67 (64‐72) | 72 (66‐78) | <.001 |
| Symptoms | |||
| Fever (≥37.3°C) | 104 (84.6) | 107 (88.4) | .376 |
| Highest temperature (°C) | 38.3 (37.8‐39.0) | 38.5 (38.0‐39.0) | .560 |
| Respiratory symptoms | 105 (85.4) | 111 (91.7) | .119 |
| Cough | 91 (74.0) | 88 (72.7) | .824 |
| Gastrointestinal symptoms | 41 (33.3) | 40 (33.1) | .964 |
| Diarrhea | 36 (29.3) | 36 (29.8) | .934 |
| Abdominal pain | 5 (4.1) | 5 (4.1) | 1.000 |
| Vital signs | |||
| SpO2, % | 97 (95‐98) | 90 (80‐96) | <.001 |
| Pulse heart rate, beats/min | 86 (80‐98) | 94 (81‐110) | <.001 |
| Respiratory rate, breaths/min | 20 (20‐21) | 25 (20‐30) | <.001 |
| Consciousness disorders | 2 (1.6) | 30 (24.8) | <.001 |
| Histories | |||
| Hypertension | 62 (50.4) | 76 (63.3) | .042 |
| Diabetes | 24 (19.5) | 27 (22.5) | .567 |
| Coronary heart disease | 15 (12.2) | 20 (16.7) | .321 |
| Previous respiratory diseases | 4 (3.3) | 20 (16.7) | <.001 |
| Durations | |||
| Time from illness onset to hospital admission, d | 11 (7‐15) | 11 (7‐15) | .871 |
| Time from illness onset to outcome, d | 32 (26‐38) | 19 (15‐25) | <.001 |
| Laboratory values | |||
| WBC count, ×109/L | 5.37 (4.43‐6.70) | 10.15 (6.20‐13.41) | <.001 |
| LYM count, ×109/L | 0.95 (0.70‐1.34) | 0.51 (0.35‐0.72) | <.001 |
| NT‐proBNP, ×102 pg/mL | 1.74 (0.75‐3.60) | 8.24 (3.50‐25.68) | <.001 |
| PCT, ng/mL | 0.05 (0.03‐0.09) | 0.36 (0.14‐1.01) | <.001 |
| hs‐TnI, pg/mL | 6.2 (3.5‐12.6) | 40.1 (12.3‐239.4) | <.001 |
| D‐dimer, μg/mL FEU | 0.79 (0.47‐1.63) | 4.16 (1.49‐21.00) | <.001 |
| ALT, U/L | 22 (14‐36) | 28 (19‐42) | .009 |
| AST, U/L | 28 (20‐39) | 45 (30‐64) | <.001 |
| Creatinine, μmol/L | 66 (54‐82) | 90 (76‐118) | <.001 |
| eGFR, mL/min/1.73 m2 | 89.0 (73.4‐94.5) | 69.9 (44.7‐81.4) | <.001 |
| hs‐CRP, mg/L | 39.8 (8.3‐79.6) | 105.4 (65.0‐165.2) | <.001 |
| ESR, mm/h | 36.0 (20.0‐59.0) | 37.0 (20.0‐60.0) | .956 |
| Serum ferritin, ×102 μg/L | 5.2 (3.9‐8.8) | 16.1 (9.7‐24.6) | <.001 |
| Interleukin‐6, pg/mL | 12.7 (3.3‐41.5) | 75.2 (35.2‐162.9) | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnI, high‐sensitivity cardiac troponin I; LYM, lymphocyte; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; PCT, procalcitonin; SpO2, oxygen saturation; WBC, white blood cell; FEU, fibrinogen equivalent units.
Data presented as median (interquartile range) and number (percentage) for continuous and categorical variables, respectively, unless otherwise indicated.
Most laboratory variables, including white blood cell (WBC) count, lymphocyte (LYM) count, amino‐terminal pro‐brain natriuretic peptide, alanine aminotransferase, aspartate aminotransferase, high‐sensitivity cardiac troponin I (hs‐TnI), D‐dimer and creatinine levels, estimated glomerular filtration rate, high‐sensitivity C‐reactive protein, procalcitonin, serum ferritin, and interleukin (IL)‐6 levels, were significantly different between the two groups. There was no statistical difference in erythrocyte sedimentation rate between the two groups (Table 1). The comparison of outcomes, clinical characteristics, and laboratory variables between older male and female patients revealed several differences (Supplementary Table S1). To eliminate bias possibly caused by sex, the clinical characteristics and laboratory variables between the two groups for male and female patients were compared separately, and the results were similar to those of all patients (Supplementary Table S2).
Statistically significant variables identified in the univariate analysis were entered into a multivariable logistic regression analysis (Table 2). Due to many missing values (>30%), serum ferritin and IL‐6 levels were excluded. Age and LYM count were significantly different (odds ratio [OR] = 1.122; 95% confidence interval [CI] = 1.007‐1.249; P = .037; and OR = 0.009; 95% CI = 0.001‐0.138; P = .001, respectively), indicating that older age and lower LYM count on admission were independently associated with increased risk for death. In addition, WBC count demonstrated a P value of .052 (OR = 1.28; 95% CI = 1.00‐1.64). The results of logistic regression and the independent risk factors (ie, age and LYM count) were used to generate ROC curves (Figure 1). The area under the ROC curve in the logistic regression model was 0.913, and that for age and LYM count were 0.653 and 0.823, respectively, indicating that LYM count and age were the most important risk factors for death.
Table 2.
Multivariate Analysis of Risk Factors for Mortality
| Risk Factor | OR | 95% CI for OR | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Demographics | ||||
| Age, y | 1.12 | 1.01 | 1.25 | .037 |
| Sex (female vs male) | 0.32 | 0.04 | 2.41 | .270 |
| Vital signs | ||||
| SpO2, % | 0.97 | 0.86 | 1.09 | .565 |
| Heart rate, beats/min | 1.00 | 0.96 | 1.04 | .977 |
| Respiratory rate, breaths/min | 1.13 | 0.94 | 1.35 | .181 |
| Consciousness disorders (disorders vs clear) | 1.38 | 0.08 | 24.00 | .827 |
| Histories | ||||
| Hypertension (yes vs no) | 0.82 | 0.24 | 2.75 | .744 |
| Previous respiratory diseases (yes vs no) | 3.24 | 0.45 | 23.57 | .245 |
| Laboratory values | ||||
| WBC count, ×109/L | 1.28 | 1.00 | 1.64 | .052 |
| LYM count, ×109/L | 0.01 | 0.00 | 0.14 | .001 |
| NT‐proBNP, ×102 pg/mL | 1.00 | 1.00 | 1.00 | .514 |
| PCT, ng/mL | 0.96 | 0.73 | 1.27 | .791 |
| hs‐TnI, pg/mL | 1.03 | 1.00 | 1.07 | .065 |
| D‐dimer, μg/mL FEU | 1.07 | 0.95 | 1.22 | .278 |
| ALT, U/L | 0.98 | 0.94 | 1.02 | .231 |
| AST, U/L | 1.04 | 0.99 | 1.09 | .137 |
| Creatinine, μmol/L | 1.04 | 0.96 | 1.13 | .340 |
| eGFR, mL/min/1.73 m2 | 1.04 | 0.92 | 1.16 | .543 |
| hs‐CRP, mg/L | 1.01 | 1.00 | 1.02 | .122 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnI, high‐sensitivity cardiac troponin I; LYM, lymphocyte; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; OR, odds ratio; PCT, procalcitonin; SpO2, oxygen saturation; WBC, white blood cell; FEU, fibrinogen equivalent units.
Figure 1.
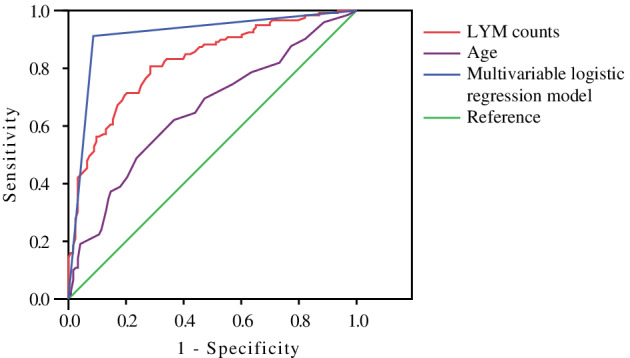
Receiver operating characteristic (ROC) curves for univariate and multivariate logistic regression models of risk factors. The purple curve is the ROC curve for the logistic regression model of age alone. The red curve is the ROC curve of the logistic regression model of lymphocyte (LYM) count alone. The blue curve is the ROC curve for the multivariable logistic regression model.
DISCUSSION
This retrospective study identified risk factors for death in hospitalized older patients with COVID‐19. Older age and lower LYM count were independent risk factors for death among older patients. In addition, higher WBC count may also be related to poorer prognosis.
Many studies have reported that age is an important risk factor for respiratory diseases.8, 9, 10 Moreover, immunosenescence has been identified as a major cause of high mortality due to severe pneumonia in older adults. 8 Humoral and cellular immune functions decline with age. Immunoglobulin M and interferon levels decrease, the number of T cells decreases, cell division and proliferation decrease, neutrophils exhibit decreased chemotaxis, and phagocytosis also decreases with age. 11 When a virus invades the body, it activates the immune system. Thus, for older patients with fewer T cells, the probability of death increases. Results of this study revealed that WBC count was higher among deceased patients than those who were discharged. A previous study reported that a decline in host immunity after viral infection may lead to secondary or bacterial infection. 12
A recent study 13 identified risk factors for death in adults who were hospitalized with COVID‐19 in Wuhan and found that older age, D‐dimer levels greater than1 μg/mL, and higher Sequential Organ Failure Assessment score on admission increased the risk for death. Additionally, severely ill patients with COVID‐19 had elevated blood levels of IL‐6, hs‐TnI, and lactate dehydrogenase, and lymphopenia. However, D‐dimer levels demonstrated no statistical difference among older patients in our study (P = .401) and may be more predictive among younger patients. Due to the absence of data, IL‐6 could not be included in the logistic regression analysis.
Fever and dry cough were the most common symptoms in patients with COVID‐19. Moreover, gastrointestinal symptoms were experienced by 33.2% of the patients in our study. For older patients with coronary heart disease and diabetes, mortality may not increase if complications are well controlled. Patients with hypertension also exhibited poorer outcomes (P = .042). A recent study reported that angiotensin‐converting enzyme 2 (ACE2) may be the host receptor for SARS CoV‐2. 14 Many models of hypertension are associated with reduced ACE2 expression, 15 indicating a plausible relationship between hypertension and COVID‐19. As commonly used antihypertensive drugs, ACE inhibitors and angiotensin II receptor blockers can upregulate ACE2 expression while reducing blood pressure.16, 17 Therefore, antihypertensive drugs should be cautiously used in patients with COVID‐19, and further studies are needed to establish the relationship between hypertension and COVID‐19. Previous respiratory disease was significantly associated with death due to COVID‐19 (P < .001), indicating that older patients with previous respiratory disease often have a poorer prognosis after infection with SARS CoV‐2. Sex was statistically different in our univariate analysis, and older men exhibited a worse outcome than older women. Some studies indicated that SARS CoV‐2 was more likely to infect males, which may be related to the high expression of ACE receptors in the lung tissues of Asian men. Other studies have suggested that the X chromosome and sex hormones may play a key role in the innate and adaptive immunity of female patients.14, 18 However, sex did not demonstrate a significant difference in the multivariate analysis in our study. Other risk factors may increase the probability of death in older men. The mean time from illness onset to hospital admission was 11 days in the two groups with different outcomes, which was also reported in another recent study. 13 Older patients who died were not denied treatment due to long wait times because there were enough wards and medical staff, which were the result of rapid actions of the government, the establishment of designated hospitals, and the national medical staff supporting Wuhan.
The present study had some limitations, the first of which was its retrospective design. More than one‐third of patients did not have laboratory data for IL‐6 and serum ferritin levels. Therefore, their roles may have been underestimated in predicting death during hospitalization. Second, the study did not include treatments such as antiviral and glucocorticoid therapy. Third, this was a single‐center study from the Sino‐French New City Branch of Tongji Hospital, which mainly admitted severe cases of COVID‐19; as such, the results may be biased.
Current treatments for COVID‐19 mainly include empirical antiviral drugs, rational glucocorticoid administration, and traditional Chinese medicine, which are effective in most patients. Currently, however, there is no proven effective drug for severely ill patients with COVID‐19. Therefore, early identification of high‐risk patients, stringent monitoring, and early intervention are needed to reduce mortality.
The COVID‐19 outbreak has been declared a global pandemic. Internationally, the number of confirmed cases and deaths is increasing rapidly, which includes numerous older patients. This study found that older age and lower LYM count on admission indicated a poorer prognosis. These older patients need to be carefully monitored and administered suitable medical interventions to reduce their mortality.
Supporting information
Supplementary Table S1: Comparison of Outcomes, Clinical Characteristics, and Laboratory Findings Between Male and Female Patients
Supplementary Table S2: Univariate Analysis, Stratified According to Sex
ACKNOWLEDGMENTS
The authors thank all patients who participated in the study and all physicians in the relevant departments.
Conflict of Interest
The authors have no conflicts.
Author Contributions
Concept and design: Dong Xu, Fangxu Tang, and Haiying Sun. Data collection: Ruoqi Ning, Yu Tao, Chong Yu, Xiaoyan Deng, Caili Zhao, and Silu Meng. Analysis and interpretation of data: Haiying Sun, Ruoqi Ning, Dong Xu, and Fangxu Tang. Manuscript preparation: All authors.
Sponsorʼs Role
None.
This article was published online on 8 May 2020. An error was subsequently identified in reference 3 and replaced with the new reference. This notice is included in the online version to indicate that it has been corrected on 7 July 2020.
Haiying Sun and Ruoqi Ning contributed equally to this work.
Contributor Information
Fangxu Tang, Email: 89650793@qq.com, Email: tfx19870521@sina.com.
Dong Xu, Email: 89650793@qq.com.
REFERENCES
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Coronavirus Disease (COVID‐19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed April 22, 2020.
- 5. National Health Commission of the Peopleʼs Republic of China . Update on COVID‐19 as of 24:00 on 5 March. http://www.nhc.gov.cn/xcs/xxgzbd/gzbd_index.shtml. Accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 6. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. 10.1007/s00134-020-05991-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chong CP, Street PR. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141‐1145. [DOI] [PubMed] [Google Scholar]
- 9. Marrie TJ. Community‐acquired pneumonia in the elderly. Clin Infect Dis. 2000;31:1066‐1078. [DOI] [PubMed] [Google Scholar]
- 10. Shu J. Pneumonia in the elderly: understanding the characteristics. South Med J. 2008;101:1086. [DOI] [PubMed] [Google Scholar]
- 11. Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heo JY, Song JY, Noh JY, et al. Effects of influenza immunization on pneumonia in the elderly. Hum Vaccin Immunother. 2018;14:744‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Qiao S, Zhang G. Analysis of angiotensin‐converting enzyme 2 (ACE2) from different species sheds some light on cross‐species receptor usage of a novel coronavirus 2019‐nCoV. J Infect. 2020;80:469‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koitka A, Cooper ME, Thomas MC, Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol. 2008;35:420‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang ML, Li X, Meng Y, et al. Upregulation of angiotensin‐converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol. 2010;37:e1‐e6. [DOI] [PubMed] [Google Scholar]
- 17. Wang W, Song A, Zeng Y, et al. Telmisartan protects chronic intermittent hypoxic mice via modulating cardiac renin‐angiotensin system activity. BMC Cardiovasc Disord. 2018;18:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Comparison of Outcomes, Clinical Characteristics, and Laboratory Findings Between Male and Female Patients
Supplementary Table S2: Univariate Analysis, Stratified According to Sex


