Abstract
Caly et al.1 reported that ivermectin inhibited severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) in vitro for up to 48 hours using ivermectin at 5 μM. The concentration resulting in 50% inhibition (IC50; 2 µM) was > 35× higher than the maximum plasma concentration (Cmax) after oral administration of the approved dose of ivermectin when given fasted. Simulations were conducted using an available population pharmacokinetic model to predict total (bound and unbound) and unbound plasma concentration‐time profiles after a single and repeat fasted administration of the approved dose of ivermectin (200 μg/kg), 60 mg, and 120 mg. Plasma total Cmax was determined and then multiplied by the lung:plasma ratio reported in cattle to predict the lung Cmax after administration of each single dose. Plasma ivermectin concentrations of total (bound and unbound) and unbound concentrations do not reach the IC50, even for a dose level 10× higher than the approved dose. Even with the high lung:plasma ratio, ivermectin is unlikely to reach the IC50 in the lungs after single oral administration of the approved dose (predicted lung: 0.0873 µM) or at doses 10× higher that the approved dose administered orally (predicted lung: 0.820 µM). In summary, the likelihood of a successful clinical trial using the approved dose of ivermectin is low. Combination therapy should be evaluated in vitro. Repurposing drugs for use in coronavirus disease 2019 (COVID‐19) treatment is an ideal strategy but is only feasible when product safety has been established and experiments of repurposed drugs are conducted at clinically relevant concentrations.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Caly et al. 1 reported that ivermectin inhibited severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) in vitro with an 50% inhibition (IC50), which was > 35× higher than the peak plasma concentration (Cmax) after oral administration of the approved dose of ivermectin.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What ivermectin dose reaches the IC50 in the lungs after oral administration in humans?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Ivermectin is unlikely to reach the IC50 in the lungs after oral administration of the approved dose or doses 10× higher than the approved doses as a single dose. The approved dose of ivermectin alone has a low probability of a success in the treatment of cornonavirus disease 2019 (COVID‐19).
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Repurposing drugs for use in COVID‐19 treatment is an ideal strategy but is only feasible when product safety has been established and experiments of repurposed drugs are conducted at clinically relevant concentrations.
Recently, an article by Caly et al. 1 reported that ivermectin inhibited severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) in vitro causing an ~ 5,000‐fold reduction in viral RNA at 48 hours with ivermectin at 5 μM. The concentration resulting in 50% inhibition (IC50) of 2 μM (1,750 ng/mL) is > 35× higher than the maximum plasma concentration (Cmax) of 0.05 µM (46.6 ng/mL) 2 after oral administration of the approved dose (~ 200 μg/kg) and ivermectin showed little to no activity 1 μM in vitro. Because ivermectin is highly bound to serum albumin (93%), 3 the IC50 is orders of magnitude higher than the unbound plasma Cmax after approved doses of ivermectin (0.0035 µM; 3.26 ng/mL).
In order to understand how in vitro SARS‐CoV‐2 inhibition by ivermectin translates to humans, one must first evaluate these concentrations compared with predicted lung concentrations in humans after oral administration of ivermectin. Theoretically, only unbound drug in the plasma could access the lungs and other tissues through passive diffusion. Ivermectin reaching the lungs after oral dosing is also likely related to lipophilicity (which is high), the low ionization at physiologic pH, the binding of ivermectin to proteins in the lungs (which is unknown), and any transporter(s) that may help maintain tissue distribution (which is unknown). Although ivermectin concentrations in lung tissue cannot be measured in humans, ivermectin exposure in homogenates obtained from the left diaphragmatic lung was reported to be 2.7× higher than total plasma exposure in cattle after a single dose. 4 Even with these higher concentrations in the lungs, ivermectin is unlikely to reach the IC50 after oral administration of the approved dose in humans.
Unlike the narrow therapeutic index for hydroxychloroquine and chloroquine, ivermectin has a wider safety margin. 2 The safety of higher doses of ivermectin has been evaluated in a phase III study, where 200–400 μg/kg doses were studied in patients with Dengue fever. 5 , 6 Even higher doses (up to 10× higher than approved doses) were studied in a small phase I trial. 7 This trial showed that ivermectin administered orally in the fasted state was well‐tolerated both after a single 120 mg dose (10× higher than approved dose) and after 60 mg three times weekly (every 72 hours). The most common adverse events were headache, nausea, dizziness, and rash. The reported incidence and type of adverse events were relatively similar between ivermectin (24%) and placebo (35%) and did not increase with dose. All dosing regimens had a mydriatic effect (the primary safety end point based on results from toxicology studies) similar to placebo. It is important to note that although this study evaluated common adverse events, the presence and incidence of rare adverse events at these high doses are unknown, given the small number of subjects studied.
The overall objective of this analysis was to evaluate what doses in humans would be predicted to result in lung concentrations reaching the IC50 in the lungs to help in designing a successful clinical trial with ivermectin in the treatment of coronavirus disease 2019 (COVID‐19).
METHODS
A population pharmacokinetic model for ivermectin reported by Duthaler et al. 8 was used in the simulations. This model was a two‐compartment model with a transit absorption model, first‐order elimination, and weight as a covariate on the central volume of distribution and clearance. This model was developed from healthy subjects with a median age of 23 years (range 20–36 years) and median weight of 64.7 kg (range 57.3–94.2 kg) receiving a single ivermectin dose of 12 mg in the fed state.
Simulations (n = 500) were performed using NONMEM version 7.4 (ICON Development Solutions, Ellicott City, MD). Total (bound and unbound) plasma concentration‐time profiles were simulated to predict exposure for the approved dose of ivermectin (200 μg/kg, in 3 mg increments) and 120 mg (studied by Guzzo et al. 7 as single doses). Because ivermectin concentrations remained steady in cattle lungs for 8 days and then declined over an additional 30 days after a single subcutaneous dose, 4 additional simulations were conducted to predict plasma concentrations with weekly dosing. In addition, 60 mg administered three times weekly was simulated (every 72 hours), given this dose was studied in healthy subjects by Guzzo et al. 7
A range of body weights in adults was sampled from the Center for Disease Control weight chart for 20‐year‐old adults, with male:female ratio being 1:1. 9 The median (3rd and 97th percentiles) weights were 70.6 kg (54.0–101 kg) in men and 58.2 kg (45.0–89.0 kg) in women, which represent most adults but does not include morbidly obese patients.
Predicted concentrations required correction for the fact that the population pharmacokinetic model was built based on subjects who received ivermectin with a high‐fat breakfast, yet ivermectin should be taken on an empty stomach. 2 Bioavailability of ivermectin is increased by 2.57‐fold increase in the fed state with no change in time of maximum plasma concentration (Tmax) and parallel concentration‐time curves 7 (suggesting a change in extent of absorption, but not rate of absorption). Therefore, all plasma concentration‐time data were divided by the geometric least squares mean ratio of the area under the curve to infinity (AUCinf) fed:fasted (2.57) to predict concentrations when ivermectin is administered in the fasted state.
Unbound plasma concentration‐time data were predicted by multiplying the total concentration by the unbound fraction in plasma (0.068).
The Cmax values for total plasma concentration were determined and multiplied by the lung homogenate:plasma ratio (2.67:1) in cattle reported by Lifschitz et al. 4 at each single dose to derive the Cmax values for total lung concentrations. The lung:plasma ratio after repeat dosing could not be determined without further modeling of the data from cattle. Some accumulation is expected in the lungs (but not plasma) with weekly or 3× weekly administration, but needs further investigation with more experimental data. A ball‐park accumulation ratio (AR) in the lungs was calculated using Eq. 1:
| (1) |
where ke is the estimated elimination rate constant from the cattle lung concentration‐time profile presented by Lifschitz et al. 4 and tau is the dosing interval.
RESULTS
Plasma ivermectin concentrations of total (bound and unbound) and unbound concentrations do not reach the IC50 reported by Caly et al. 1 even for dose level 10× higher than the approved dose, or after repeat dosing (Figure 1 ). Plasma exposures did not increase substantially after repeat dosing, with very limited ivermectin accumulation in plasma after three times weekly or weekly dosing.
Figure 1.
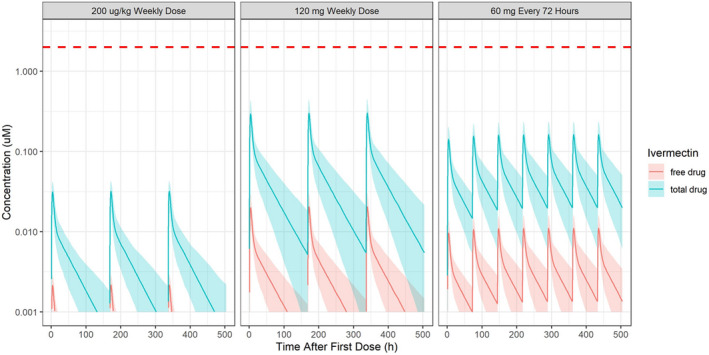
Total (bound and unbound) and unbound plasma ivermectin concentrations over time relative to the 50% inhibition (IC50) after approved doses of ivermectin (200 µg/kg) administered once weekly (instead of as a single dose), and after higher doses, including 60 mg every 3 days or 120 mg once weekly. Red dashed line = IC50 reported by Caly et al. (2020), 2 µM (1750 ng/mL). Blue shaded area and line = total plasma drug concentration and its 95% confidence interval (CI); red shaded area and line = free plasma drug concentration and its 95% CI.
Even with the high lung homogenate:plasma ratio, ivermectin is unlikely to reach the IC50 of 2 µM in the lungs after single oral administration of the approved dose (predicted lung concentration: 0.0873 µM) or at doses 10× higher that the approved dose administered orally (predicted lung concentration: 0.820 µM; Table 1 ). Currently, there is no lung tissue disposition data available after repeated dosing, but the ball‐park accumulation ratio in the lungs can be used to predict lung concentrations relative to the IC50 after repeat dosing. If the approved dose was administered weekly, the ball‐park accumulation ratio in lung tissue is 1.3, which would result in lung concentrations that are 1/20th of the IC50. If the approved dose was administered three times weekly, the ball‐park accumulation ratio in lung tissue is 2.2, which would result in lung concentrations that are 1/10th of the IC50. If the approved dose was administered daily, the ball‐park accumulation ratio in lung tissue is 5.35, which would result in lung concentrations that are 1/4th of the IC50. Using the ball‐park accumulation ratio for higher doses, predicted lung concentrations would be ~ 1/5th the IC50 after 60 mg three times weekly or after 120 mg once weekly.
Table 1.
Predicted maximum total plasma concentrations and lung concentrations after various doses of ivermectin administered fasted
| Treatment |
Predicted total Cmax (µM) Median [2.5th, 97.5th percentiles] |
|
|---|---|---|
| Plasma | Lung b | |
| Single dose | ||
| 200 µg/kg single dose, labeled dose | 0.0327 [0.0228, 0.0429] | 0.0873 [0.0609, 0.115] |
| 120 mg single dose a | 0.307 [0.204, 0.449] | 0.820 [0.545, 1.20] |
| Repeated dose, 3 weeks | ||
| 200 µg/kg weekly | 0.0334 [0.0230, 0.0439] | |
| 60 mg every 72 hours a | 0.169 [0.113, 0.248] | |
| 120 mg weekly | 0.313 [0.207, 0.462] | |
Cmax, peak plasma concentration.
Each administered to 12 subjects (Guzzo et al., 2002).
Calculated based on reported lung:plasma ratio of 2.67 in cattle (Lifschitz et al., 2000).
DISCUSSION
The in vitro studies showing that ivermectin inhibited SARS‐CoV‐2 1 were conducted at concentrations that were substantially higher than predicted plasma and lung concentrations in humans receiving the approved dose of ivermectin. Therefore, the likelihood of a successful clinical trial using the approved dose of ivermectin is low. If a clinical trial is conducted, a well‐controlled dose‐response study should be considered and the feasibility of ivermectin as an inhaled treatment should be evaluated.
A first step would be to conduct the in vitro study reported by Caly et al. 1 evaluating whether other antivirals can potentiate ivermectin’s inhibition of SARS‐COV‐2 to identify whether an ivermectin concentration of 0.1 μM (rather than 5 μM) with a concomitant antiviral can inhibit SARS‐COV‐2. Repurposing drugs for use in the treatment of COVID‐19 is an ideal strategy but is only feasible if the safety of the product use has been established at the dose levels that produce efficacy. Therefore, in vitro experiments of repurposed drugs should be conducted at clinically relevant concentrations.
As soon as the in vitro findings were published, clinicians all over the world were using ivermectin off‐label. Patel et al. 10 reported this week through an observational registry‐based study from 169 hospitals across the world that ivermectin 150 μg/kg administered to 52 patients after institution of mechanical ventilation showed a potential decrease in hospital stay length and survival benefit compared with 1,918 conventionally treated patients. 10 It is noted that these results did not account for the comorbidities that might account for these differences. Nevertheless, if ivermectin did contribute to these clinical findings, it would suggest that the in vitro findings by Caly et al. 1 do not correlate with the very small amounts of the drug in lung tissue in humans, concentrations in lung homogenate do not correlate with concentrations at the site of action or those expected based on ivermectin’s large volume of distribution, concentrations of the drug do not need to reach the IC50 for clinical benefit (i.e., the IC50 is not relevant), distribution into or retention in the lung tissue of humans is greater than in cattle, or that accumulation in lung tissue is much greater (> 20‐fold) than expected after repeat dosing. Given that lung tissue:plasma concentration ratio in goats (3× at 2 and 7 days after oral administration) was similar to cattle and lung tissue:plasma concentration ratio in mice (1.4× 24 hours after oral administration) was slightly lower, excessive accumulation of ivermectin in human lungs is unlikely. 11 , 12
If a clinical study is conducted with ivermectin, it would be important to conduct a well‐controlled clinical dose‐response study with ivermectin at a low dose (the approved dose, with lower likelihood of success) and at a higher dose relative to placebo in patients with COVID‐19. The ideal higher dose of ivermectin has not been established. Ivermectin doses up to 120 mg have only been administered to a small number of subjects. 7 After daily dosing of the ivermectin 200 μg/kg, lung concentrations are predicted to be around 1/4th of the IC50. Daily dosing of ivermectin at the approved dose for longer periods (e.g., 14 days) have only been studied in small studies for serious infections where an unapproved subcutaneous formulation was used. 13 Therefore, if higher doses are studied weekly, if the approved dose is studied daily, or a parenteral formulation is used, subjects will need to be monitored closely.
Ivermectin is extensively metabolized by CYP3A4 to numerous metabolites and is a substrate for P‐glycoprotein. Less than 1% of the ivermectin dose is eliminated unchanged in the urine. Thus, any study would need to control for factors affecting variability in the exposure to ivermectin, including administering the dose in the fasted state and excluding P‐glycoprotein and CYP3A4 inhibitors, 2 which could increase ivermectin exposure. The pharmacokinetics of ivermectin in elderly patients have not been reported. Theoretically, metabolism may decrease with age resulting in a higher exposure to ivermectin in elderly patients as well.
Lastly, a potential longer‐term solution would be to consider whether inhaled treatment with ivermectin is feasible. Inhaled treatment would allow for higher concentrations at the site of action while limiting the systemic exposure, but may require further study of the safety and tolerability in animals prior to human exposure. Only one nonclinical study has been published on inhaled ivermectin in Sprague Dawley rats, in which the no‐observed‐adverse‐effect level (NOAEL) after 28 days of inhaled ivermectin was identified to be 380 mg/m3, 14 and no studies using the inhaled route of administration have been identified in humans. Of key importance is determining whether ivermectin has general properties that would allow inhalation, with no local tolerability issues. Experts must, therefore, evaluate whether ivermectin possesses the ideal properties for inhalation, and whether inhalation of ivermectin poses any theoretical risks that might limit this route of administration.
Overall, the results identified in the paper by Caly et al. 1 create an opportunity for interdisciplinary collaboration in helping to understand the highest probability of success for ivermectin treatment, prior to exploration in clinical studies (or worse yet, off‐label use by the general public) with a less‐than‐ideal dose.
Funding
No funding was received for this work.
Conflict of Interest
V.S., L.L., and J.Z. are consultants and have worked with many companies but have not worked with companies who produce ivermectin orally (EDENBRIDGE PHARMS and MERCK). All authors declared no competing interests for this work.
Author Contributions
G.S., J.Z., and L.L. wrote the manuscript. G.S. designed the research. J.Z. performed the research. J.Z. and L.L. analyzed the data.
Acknowledgment
The authors thank Anginelle Alabanza, MS, RAC, for her help reviewing and editing the manuscript.
References
- 1. Caly, L. , Druce, J.D. , Catton, M.G. , Jans, D.A. & Wagstaff, K.M. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 178, 104787 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. STROMECTOL® (ivermectin) tablets [package insert]. Merck Sharp & Dohme Corp; <https://www.merck.com/product/usa/pi_circulars/s/stromectol/stromectol_pi.pdf> (Revised 2018). [Google Scholar]
- 3. Klotz, U. , Ogbuokiri, J.E. & Okonkwo, P.O. Ivermectin binds avidly to plasma proteins. Eur. J. Clin. Pharmacol. 39, 607–608 (1990). [DOI] [PubMed] [Google Scholar]
- 4. Lifschitz, A. et al. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet. Parasitol. 87, 327–338 (2000). [DOI] [PubMed] [Google Scholar]
- 5. NCT02045069 . Efficacy and Safety of Ivermectin Against Dengue Infection <https://clinicaltrials.gov/ct2/show/NCT02045069> (2015).
- 6. Efficacy and Safety of Ivermectin against Dengue Infection: A Phase III, Randomized, Double‐blind, Placebo‐controlled Trial. The 34th Annual Meeting The Royal College of Physicians of Thailand.
- 7. Guzzo, C.A. et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 42, 1122–1133 (2002). [DOI] [PubMed] [Google Scholar]
- 8. Duthaler, U. et al. Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers. Br. J. Clin. Pharmacol. 85, 626–633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . United States Growth Charts. (National Center for Health Statistics, 2009). [Google Scholar]
- 10. Patel, A. Ivermectin in COVID‐19 related critical illness. SSRN Electronic J. <https://ssrn.com/abstract=3580524> (2020). [Google Scholar]
- 11. Lespine, A. , Alvinerie, M. , Sutra, J.F. , Pors, I. & Chartier, C. Influence of the route of administration on efficacy and tissue distribution of ivermectin in goat. Vet. Parasitol. 128, 251–260 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Schinkel, A.H. et al. Disruption of the mouse mdr1a P‐glycoprotein gene leads to a deficiency in the blood‐brain barrier and to increased sensitivity to drugs. Cell 77, 491–502 (1994). [DOI] [PubMed] [Google Scholar]
- 13. Turner, S.A. , Maclean, J.D. , Fleckenstein, L. & Greenaway, C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am. J. Trop. Med. Hygiene 73, 911–914 (2005). [PubMed] [Google Scholar]
- 14. Ji, L. et al. Study on the subacute inhalation toxicity of ivermectin TC in rats. Chinese J. Comparat. Med. 26, 70–74 (2016). [Google Scholar]


