To the Editor
Patients with coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus are at risk for acute kidney injury (AKI). Requirements of kidney replacement therapies (KRT) may be particularly intense for kidney transplant recipients with COVID‐19 who develop AKI, due to their chronic use of steroids [1] and theoretically higher viral loads [2] that may lead to a catabolic state [3]. Herein, we report the case of a kidney transplant recipient with COVID‐19 infection, AKI, and a high catabolic state evidenced by refractory hyperkalemia and azotemia.
The patient is a 44‐year‐old male who received a deceased donor kidney transplant 7 years prior to presentation. Maintenance immunosuppression consisted of tacrolimus, prednisone, and mycophenolate sodium. He presented with dyspnea (oxygen saturation of 91% and respiratory rate of 24 breaths per minute) multifocal opacities on chest radiograph, and a nasopharyngeal swab positive for SARS‐CoV‐2 (RT‐PCR). Upon admission, immunosuppressive therapy was maintained (tacrolimus target was lowered to 4 ng/ml) and he did not receive hydroxychloroquine due to prolonged QTc interval. He was not on renin–angiotensin system (RAS) inhibitors. On admission, his serum creatinine was at his baseline of 2.3 mg/dl. D‐Dimer was 1.1 µg/ml FEU (peak 19.3 µg/ml FEU on day 12), lactate dehydrogenase 285 U/l (peak 1230 U/l on day 11), and ferritin 373 ng/ml (peak 4292 ng/ml on day 9). Arterial lactate remained within normal limits (0.5–1.99 mmol) during the hospital course. The troponin level was 0.3 ng/ml upon admission, and subsequently decreased. On day 2 of admission, he developed AKI, with a peak creatinine of 5.9 mg/dl, potassium 6.0 meq/l, and blood urea nitrogen (BUN) 144 mg/dl on day 11 requiring hemodialysis. On day 10, the patient was intubated for respiratory failure and methylprednisolone (40 mg three times daily) was administered from day 11 through day 15, and then tapered to twice daily. The patient did not require vasopressor support. While intubated, tube feeds were administered.
Between day 14 and day 17, the patient required daily hemodialysis treatments for refractory hyperkalemia and azotemia, with a potassium of 7.1 meq/l despite treatment with sodium zirconium cyclosilicate 10 g three times daily and loop diuretics. After four hemodialysis treatments, phosphorus remained elevated (11.7 mg/dl). Urine output decreased from 4.1 l/24 h on day 14 to 240 ml/24 h on day 17. Dialysis treatments were increased in intensity, with blood flow rates up to 500 cc/min through a well‐functioning arteriovenous fistula (AVF), use of a high‐flux dialysis filter, and potassium‐free dialysate (Fig. 1). At the present time (day 31), the patient remains both dialysis‐ and ventilator‐dependent.
Figure 1.
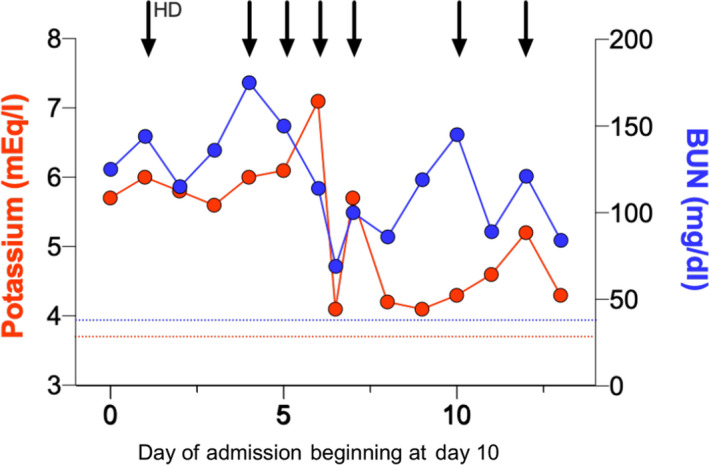
Changes in serum potassium and blood urea nitrogen (BUN) over time, starting at day 10 of admission. Arrows indicate the intermittent hemodialysis sessions. The dotted lines illustrate baseline values (day 1).
Persistently elevated serum potassium, BUN, and phosphorus levels in this patient with COVID‐19 infection indicate a catabolic state. Though the AVF appeared to be well functioning, it is possible that poor blood flow through the vascular access contributed to azotemia. Perfusion seemed to be preserved given the absence of vasopressor use and normal lactate levels. Hemodialysis was required to correct severe electrolyte derangements associated with AKI and catabolic syndrome. There was no evidence of hemolysis or rhabdomyolysis (creatine phosphokinase, 215 U/l; haptoglobin, 272 mg/dl; and total bilirubin, 1.4 mg/dl).
Viruses can drastically alter metabolism of infected cells in an effort to replicate and spread, and may activate catabolic pathways including glycolysis, fatty acid synthesis, and glutaminolysis [4]. Cell catabolism leads to severe electrolyte derangements that can be challenging to manage, particularly in patients with AK when KRT are limited. Recent data have shown that similar to other coronaviruses, SARS‐CoV‐2 alters central cellular pathways and thus increases cellular metabolism [5, 6]. More data are needed to better understand SARS‐CoV‐2‐induced changes in cellular metabolism, as these may lead to effective therapies. To the best of our knowledge, this is the first case of a COVID‐19 kidney transplant recipient with such a severe catabolic state. While catabolic state is common in patients with multiorgan failure, we contend that unique characteristics of SARS‐CoV2 make this condition particularly severe. A better understanding of the pathophysiology of this condition may help improving outcomes for this critically ill patient population.
Conflicts of interest
The authors of this manuscript have no conflicts of interest to disclose as described by Transplant International.
References
- 1. Fernández‐Cabezón L, Galán B, García JL. New insights on steroid biotechnology. Front Microbiol 2018; 9: 958. 10.3389/fmicb.2018.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fishman JA, Grossi PA. Novel Coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant 2020. 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burgner A, Ikizler TA, Dwyer JP. COVID‐19 and the inpatient dialysis unit: managing resources during contingency planning pre‐crisis. CJASN 2020; 15: 720. Available from: https://cjasn.asnjournals.org/content/early/2020/04/03/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanchez EL, Lagunoff M. Viral activation of cellular metabolism. Virology 2015; 479–480: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denise B, Kevin K, Benjamin K, Widera M. SARS‐CoV‐2 infected host cell proteomics reveal potential therapy targets. 2020. Available from: https://www.researchsquare.com/article/rs‐17218/v1[cited 2020 April 11].
- 6. Wu Q, Zhou L, Sun X, et al. Altered Lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep 2017; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]


