Summary
The current international COVID‐19 health crisis underlines the importance of adequate and suitable personal protective equipment for clinical staff during acute airway management. This study compares the impacts of standard air‐purifying respirators and powered air‐purifying respirators during simulated difficult airway scenarios. Twenty‐five anaesthetists carried out four different standardised difficult intubation drills, either unprotected (control), or wearing a standard or a powered respirator. Treatment times and wearer comfort were determined and compared. In the wearer comfort evaluation form, operators rated mobility, noise, heat, vision and speech intelligibility. All anaesthetists accomplished the treatment objectives of all study arms without adverse events. Total mean (SD) intubation times for the four interventions did not show significant differences between the powered and the standard respirator groups, being 16.4 (8.6) vs. 19.2 (5.2) seconds with the Airtraq™; 11.4 (3.4) vs. 10.0 (2.1) seconds with the videolaryngoscope; 39.2 (4.5) vs. 40.1 (4.8) seconds with the fibreoptic bronchoscope scope; and 15.4 (5.7) vs. 15.1 (5.0) seconds for standard tracheal intubation by direct laryngoscopy, respectively. Videolaryngoscopy allowed the shortest intubation times regardless of the respiratory protective device used. Anaesthetists rated heat and vision significantly higher in the powered respirator group; however, noise levels were perceived to be significantly lower than in the standard respirator group. We conclude that standard and powered respirators do not significantly prolong simulated advanced intubation procedures.
Keywords: CBRN, COVID‐19, difficult airway management, personal protective equipment, SARS‐CoV‐2
요약
현재 COVID‐19에 따른 국제적 보건 위기는 급성 기도 관 리 중 임상 교원을 위한 적절하고 적합한 개인 보호장비의 중 요성을 강조한다. 본 연구에서는 시뮬레이션한 어려운 기도 시 나리오에서 표준 공기 정화 호흡보호구와 전동식 공기 정화 호흡보호구의 영향을 비교하였다. 25명의 마취과 의사는 비보 호군(대조군), 표준 호흡보호구 또는 전동식 호흡보호구 착용 군으로 배정되어 네 가지 표준화된 어려운 기도 삽관 훈련을 수행하였다. 치료시간과 착용자의 편안함을 결정하고 비교하 였다. 착용자의 편안함 평가 양식에서는 이동성, 소음, 열, 시 각 및 말 명료도(speech intelligibility)를 평가하였다. 모든 마 취과 의사는 부작용 없이 모든 군의 치료 목표를 달성하였다. 네 가지 중재에 대한 총 평균(SD) 기도 삽관 시간은 전동식 호 흡보호구군 및 표준 호흡보호구군에서 유의한 차이를 보이지 않았다. AirtraqTM 사용시, 16.4초(8.6) vs. 19.2초(5.2); 비디오 후두경 사용시 11.4초(3.4) vs. 10.0초(2.1); 굴곡기관지경 사용 시, 39.2초(4.5) vs. 40.1초(4.8); 후두경을 사용한 표준 기도 삽 관시 15.4초(5.7) vs. 15.1초(5.0). 비디오후두경의 경우 사용하 는 호흡기 보호장치에 상관없이 기도 삽관 시간이 가장 짧았 다. 마취과 의사는 열과 시각을 전동식 호흡보호구군에서 유 의하게 높게 평가하였지만 소음 수준은 표준 호흡보호군보다 유의하게 낮다고 인식하였다. 저자들은 표준 호흡보호구 및 전동식 호흡보호구가 시뮬레이션된 어려운 기도 삽관을 유의 하게 연장하지 않는다고 결론지었다.
Introduction
Airway management in critically ill patients contaminated with chemical, biological radiological or nuclear substances requires personal protective equipment (PPE) [1, 2]. The past two decades have focused on the hazards for frontline medical staff posed by the deliberate release of weaponised chemical, biological, radiological or nuclear substances. The occupational hazards of healthcare workers during naturally occurring pandemics, however, have a much longer history. Bio‐aerosol infection risks to healthcare professionals may arise from direct patient contact, but are especially high during aerosol‐generating procedures such as: intubation; bronchoscopy; non‐invasive ventilation; high‐frequency oscillating ventilation; induction of sputum; and surgical procedures involving high speed devices. Notably, during the 2003 severe acute respiratory distress syndrome pandemic, 21% of those who had the disease were healthcare workers [3]. In Canada, this was reported as 43%, with anaesthetists being amongst the highest risk group [3, 4]. Public Health England [5] and the World Health Organization [6] provide guidelines for frontline medical staff regarding the adequate levels of PPE required for chemical, biological, radiological or nuclear incidents, all recently updated during the current coronavirus disease 2019 (COVID‐19) pandemic.
Patients affected by high‐consequence infectious diseases need to be isolated, and medical personnel require adequate and continuous respiratory, cutaneous and eye protection whilst caring for them. Certain medical procedures or treatments carry a higher risk of pathogen transmission; these aerosol‐generating procedures are typically encountered by paramedics, anaesthetists and intensivists during airway procedures and require particular strategies for management [7]. Advanced life support and tracheal intubation in the emergency department, or on a medical ward, can be very challenging even without respiratory protective equipment. Previous studies by our group have highlighted the problems and pitfalls of advanced life support in chemical, biological, radiological or nuclear environments [8, 9, 10, 11]. The most commonly used respiratory protection devices are standard respirators, either designed as a half mask or as a full facepiece. A full facemask covers the eyes, nose, mouth and chin; it seals against the face of the wearer and is held in place by adjustable straps. When used in a filtering device, air is drawn into the mask through a filter either when the wearer breathes in, or from a power‐assisted filtering device [12]. The visor provides protection against particulates, splashes and gases, yet should allow good visibility for the wearer. A half mask respirator is a facepiece which only covers the nose, mouth and the chin of the wearer, held in place with adjustable straps [12]. Half masks do not have a visor for eye protection and provide lower assigned protection factor levels than full facemasks [13].
In recent years, powered air‐purifying respirators have been introduced; a complete powered filtering device consisting of a battery‐operated turbo unit, a filter and a loose‐fitting headtop, for example, a hood or visor [12]. Although they are bulkier and more expensive, powered respirators eliminate the need for fit testing and problems of heat build‐up, dead‐space ventilation and airflow resistance [12, 13, 14]. This is the first study comparing the impact of modern powered respirators and standard respirators on simulated difficult airway procedures. The primary outcome measure of this study was as the difference in intubation times for various airway management procedures, with wearer comfort a secondary outcome.
Methods
The study received Guy’s and St Thomas’ NHS Foundation Trust Research and Development approval. The study did not require review by a research ethics committee, as the research only involved staff as participants.
The objective was to recruit 25 anaesthetists within our hospital Trust whose duties involved responding to trauma calls. Exclusion criteria for the participants included those suffering from asthma, claustrophobia or a history of panic disorder.
Twenty‐five subjects gave written and informed consent after receiving a detailed explanation of the treatment protocol and formal face‐to‐face training in the use of personal protective equipment. All volunteers had been instructed they could withdraw from the study at any time if they wished. The study started in December 2019 and finished in February 2020.
All participants wore a long‐sleeved surgical gown and gloves during the procedures.
The powered respirator used the 3M Scott‐Duraflow platform (Powered Air‐Purifying Respirator, 3M Scott Safety Ltd, West Pimbo, Skelmersdale, UK) (Fig. 1). As a headtop, a loose fitting flow‐hood was used which was connected via a corrugated hose. The fan‐unit, weighting 1.4 kg with filters and battery, provides a guaranteed airflow of 160 l.min−1, has a battery operating time of 8 h from a single charge and has a sound level of less than 70 dB. The standard respirator was the First Responder Respirator (FRR, 3M Scott Safety Ltd, West Pimbo, Skelmersdale, UK) (Fig. 2). This full‐face respirator is the civilian version of the UK Ministry of Defence General Service respirator approved to the regulatory standards of EN136 Class 3 and BS8468‐2 for use in a chemical, biological, radiological or nuclear environment. The FRR it weighs 640 g, has a panoramic visor and has re‐breathed carbon dioxide levels significantly below 1%.
Figure 1.
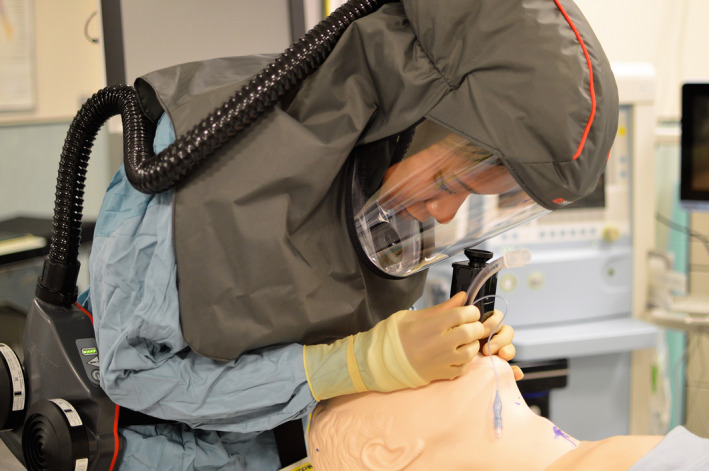
Powered air‐purifying respirator with hood.
Figure 2.
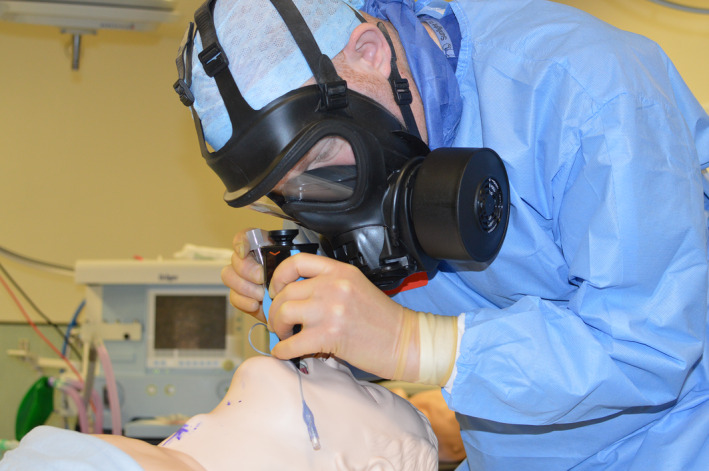
Standard air‐purifying respirator.
Both respiratory protective devices used the 3M™ Scott™ CFR32 CBRN A2B2E2K2‐P3 R filter, with its EN148‐1 compliant 40‐mm thread, designed for use with defence and public safety respirators. The filter is suitable for military and ‘first responder’ applications. It protects against toxic industrial chemicals and chemical, biological, radiological and nuclear warfare agents, including: gases and vapours from organic compounds with a boiling point above 65ºC; inorganic gases and vapours; solid and liquid toxic and radioactive particulates; and micro‐organisms, for example, bacteria and viruses. The third ensemble, which was used as a control, was standard operating department attire and a long‐sleeved surgical gown. The airway scenarios were carried out on a Laerdal Airway Management Trainer™ (Laerdal Medical Ltd, Orpington, UK). The manikin was placed on a standard stretcher, and the scenarios were carried out in one of our main operating theatres (in‐situ simulation).
The airway management protocol was guided by the Difficult Airway Society guidelines for the management of unanticipated difficult intubation [15] and compliant with our local guidelines and the routine airway management equipment used in our hospital. Participants undertook the tasks in all three protection levels (control, plus the two with respiratory protective equipment). The airway tasks remained identical in each group; however, we randomised the order in which the two types of respiratory protective equipment and standard operating department attire was worn, to counter any learning effects. The order of respirator/standard theatre wear use was determined by opening sealed envelopes before starting the simulation. The investigator was blinded to the contents of the envelope and each envelope was externally identical. All volunteers were briefed on the scenario and the sequence of the tasks, and received formal training in the respiratory protective equipment used.
A conventional laryngoscope with a size 3 Macintosh blade was used in the control group and a standard Airtraq™, size green, using the eyepiece, was the device used for indirect tracheal intubation in the Airtraq group (Airtraq, Getxo, Spain). For the videolaryngoscopy group, the standard Airtraq mounted with the Airtraq A‐390 camera was used. The Ambu aScope 4 regular bronchoscope was used for the fibreoptic intubation group (Ambu A/S, Ballerup, Denmark).
Times for the completion of each airway management procedure were measured with a stopwatch which was started after the investigator commanded the specific task to commence. The stopwatch was then stopped after the verbal confirmation by the participant that the tube had been correctly placed. Correct tube placement was confirmed by the investigator. After completing all scenarios, participants were asked to complete a wearer comfort evaluation form to rate their perceptions of mobility, noise, heat, vision and speech intelligibility of each respiratory protection system used, on a 5‐point scale where 0 represented the worst conditions, and 5, the best (see also supporting information, Appendix S1).
The required sample size had been established by our previous studies using the same model [6, 7, 8, 9]. Our power calculation was based on a study that measured a standardised resuscitation scenario using powered respirator hoods [9]. The statistical analysis was performed using SPSS v26.0 (IBM, Armonk, New York, NY, USA). Continuous data were normality tested using the Shapiro–Wilk test; this was followed up by multiple comparisons of the time periods by analysis of variance (ANOVA). Data from the 5‐point scale wearer comfort evaluation form were analysed with the Mann–Whitney test. A p value of < 0.05 was considered statistically significant.
Results
Within the study period, we were able to recruit 25 volunteers. All anaesthetists participating in the study successfully accomplished the treatment objectives of all the study arms. All interventions ended with successful tracheal tube placement. The treatment times are displayed in Table 1.
Table 1.
Treatment times (s) of the individual airway management tasks. Values are mean (SD).
| Control | Powered respirator | Standard respirator | p value* | |
|---|---|---|---|---|
| Airtraq intubation | 14.5 (5.5) | 16.4 (8.6) | 19.2 (5.2) | 0.167 |
| VL intubation | 11.5 (4.7) | 11.1 (3.4) | 10.0 (2.1) | 0.187 |
| FO intubation | 32.4 (4.8) | 39.2 (4.5) | 40.1 (4.8) | 0.977 |
| Direct laryngoscopy (control) | 12.9 (4.7) | 15.7 (5.9) | 15.1 (5.0) | 0.833 |
FO, fibreoptic; VL, videolaryngoscopy.
p values are for comparisons between the two respiratory protective equipment groups.
Intubation times were significantly shorter when using videolaryngoscopy, regardless of the respiratory protective device used. Airway management times of the Airtraq group, the videolaryngoscopy and the fibreoptic intubation group were independent of the respiratory protective device used. Mean fibreoptic intubation times were significantly longer compared to Airtraq, videolaryngoscopy and standard laryngoscopy times. Anaesthetists rated their personal sensation of heat build‐up and perceived vision significantly higher in the powered respirator group; however, noise levels scored significantly lower compared to the standard respirator group (Table 2).
Table 2.
Wearer comfort evaluation form ratings. Values are median (IQR [range]).
| Control | Powered respirator | Standard respirator | p value* | |
|---|---|---|---|---|
| Mobility | 5 (0 [0]) | 3 (2‐4 [1–4]) | 3 (2‐4 [1–5]) | 0.601 |
| Noise | 5 (0 [0]) | 2 (1‐3 [1–4]) | 3 (2‐4 [1–5]) | 0.021 |
| Heat | 5 (0 [0]) | 3 (3‐4 [2–5]) | 2 (1‐2 [0–4]) | 0.002 |
| Vision | 5 (0 [0]) | 3 (2‐4 [1–4]) | 2 (1‐3 [1–5]) | 0.008 |
| Speech intelligibility | 5 (0 [0]) | 2 (1‐4 [0–5]) | 3 (2‐4 [1–5]) | 0.062 |
p values are for comparisons between the two respiratory protective equipment groups.
Discussion
Employers in the UK have a legal responsibility to control substances hazardous to health in the workplace, and to prevent and adequately control their employees’ exposure to those substances [13]. In the event of a chemical or radiological incident, casualties are a potential source of dangerous contamination to healthcare workers and should therefore be decontaminated before hospital admission. However, patients affected by biological pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) present frontline medical staff with significantly different challenges: there is no immediate point of care detection by monitors of radiation or chemical agents, and these patients will continue to shed their pathogens during their hospital stay. It has been reported that some of the anaesthetists who contracted severe acute respiratory syndrome during the 2003 pandemic were only wearing standard surgical facemasks while they performed tracheal intubation on infected patients [4]. Reports from the current COVID‐19 pandemic in Italy stress the importance of respiratory protective equipment for healthcare workers in anaesthetics and intensive care [16]. Recent studies looking at the impact of PPE on advanced life support and airway management have compared the effect of different standard respirators and powered respirators [8, 9, 10, 11, 17, 18] but this is the first study to compare the use of modern respirators and powered respirators during advanced airway management procedures. Our main finding was that the use of videolaryngoscopy for difficult airway management proved to have certain advantages whilst wearing respiratory protection. Videolaryngoscopy is already widely advocated under normal circumstances [19] but also allows the anaesthetist to keep their head further away from the patient during airway management in COVID‐19 patients [7, 20].
Fibreoptic intubation during our study needed significantly more time than direct laryngoscopy or videolaryngoscopy. However, neither the standard nor the powered respirators prolonged fibreoptic intubation in our model.
The powered respirator ensemble scored significantly better in user rating for heat and vision. The positive ratings of powered respirators for temperature and heat build‐up are consistent with previous reports [10, 21, 22]; as a result, many healthcare workers favour the use of powered respirators over tight fitting masks. In addition, the participants scored the standard respirators significantly higher on visual clarity. This might be due to the larger visor of the powered respirators and the inherent capability of a powered respirator to compensate for air leaks while wearing spectacles. In contrast, as standard respirators lack the noise of the powered air‐purifying respirator’s internal fan module, participants favoured the standard respirator for noise. In our study, both respiratory protective devices were found to impair speech intelligibility. The facepieces used in air‐purifying respirators are known to significantly reduce sound transmission by attenuating and distorting sound and by restricting lower jaw movement [14, 23].
Two aspects which fell outside the scope of our study should be considered. The first is cost; the standard respirator used in this study is 70% cheaper than the powered respirator. The second is decontamination and disinfection. Disinfection of all the different parts of a powered respirator takes significantly longer than cleaning a single respirator facemask. Further, although powered respirators have been preferred by a lot of healthcare workers [10, 21], their weight, bulk and connection to the corrugated breathing tube may impede the mobility of the wearer to a certain extent. It must also be noted that if the air supply to the powered respirator fails, then these devices will not provide any protection to the wearer. The user will then be exposed to contaminants in the ambient air, and increased levels of carbon dioxide due to rebreathing [12]. Therefore it is prudent to have charged, spare batteries readily available. Finally, we should note that our study took place in a controlled, simulated environment, and the participants were not required to physically exert themselves, and therefore we are unable to comment on heat build‐up and the effects of standard respirators’ breathing resistance over time.
The Health and Safety at Work etc Act 1974 and the Management of Health and Safety at Work Regulations 1999 require the provision of adequate and suitable respiratory protective equipment [24, 25]. During the current COVID‐19 pandemic our hospital, which is a high consequence infectious diseases centre, has found the use of powered respirators most suitable for prolonged airway or surgical procedures and for members of staff who have failed their fit‐testing. Quantitatively fit‐tested tight‐fitting standard respirators, mainly as half masks, are, however, the main respiratory protective device used by our anaesthetic and operative department staff. Nevertheless, an understanding of the effect of the different protective devices allows for appropriate safe use in clinical practice.
Supporting information
Appendix S1 Wearer comfort evaluation form.
Acknowledgements
This study was prospectively registered by the UK Clinical Research Network (UKCRN) under the portfolio ID 204282. The authors wish to thank Professor D. Crouch (3M Global Subject Matter Expert ‐ Application Engineering, Defence and Public Safety) for the provision of free samples of respiratory protective equipment. This work was supported by the Department of Anaesthesia, Guy’s and St Thomas´ NHS Hospital Trust, London, UK and the NIHR GSTFT/KCL Biomedical Research Centre, Guy's Hospital, London. No other external funding or competing interests declared.
References
- 1. World Health Organization . Public health response to biological and chemical weapons. 2004. http://www.who.int/csr/delibepidemics/biochemguide/en/ (accessed 19/04/2020).
- 2. Schumacher J. Respiratory protection for medical first responders and receivers. In: Racz L, Yamamoto D, Eninger R, eds. Handbook of Respiratory Protection. Boca Raton, FL: Taylor and Francis, 2018: 72–91. [Google Scholar]
- 3. World Health Organization . Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2004. https://www.who.int/csr/sars/country/table2004_04_21/en (accessed 19/04/2020).
- 4. Kamming D, Gardam M, Chung F. Anaesthesia and SARS. British Journal of Anaesthesia 2003; 90: 715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Public Health England . Guidance on infection prevention and control for COVID‐19. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control (accessed 19/04/2020).
- 6. World Health Organization: Coronavirus disease (COVID‐19) technical guidance: Infection prevention and control. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/infection‐prevention‐and‐control (accessed 19/04/2020).
- 7. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19. Anaesthesia 2020;75:785‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schumacher J, Arlidge J, Garnham F, Ahmad I. A randomised crossover simulation study comparing the impact of chemical, biological, radiological or nuclear substance personal protection equipment on the performance of advanced life support interventions. Anaesthesia 2017; 72: 592–7. [DOI] [PubMed] [Google Scholar]
- 9. Schumacher J, Gray SA, Michel S, Alcock R, Brinker A. Respiratory protection during simulated emergency pediatric life support. Prehospital and Disaster Medicine 2013; 28: 33–8. [DOI] [PubMed] [Google Scholar]
- 10. Schumacher J, Gray SA, Weidelt L, Prior K, Brinker A, Stratling WM. Comparison of powered and conventional air‐purifying respirators during simulated resuscitation of CBRN victims. Emergency Medical Journal 2009; 26: 501–5. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher J, Runte J, Brinker A, Prior K, Heringlake M, Eichler W. Respiratory protection during high fidelity simulated resuscitation of casualties contaminated with chemical warfare agents ‐ Respiratory protection for anaesthetists during resuscitation. Anaesthesia 2008; 63: 593–8. [DOI] [PubMed] [Google Scholar]
- 12. British Standards . BS EN 529:2005 Respiratory protective devices — Recommendations for selection, use, care and maintenance — Guidance document. Technical Committee PH/4, Respiratory protection, to Subcommittee PH/4/11. 2005. https://www.breathesafety.com/files/EN5292005RespiratoryProtectiondevices.pdf (accessed 19/04/2020).
- 13. Health and Safety Executive . Respiratory protective equipment at work ‐ A practical guide. HSG53. 4th edn. 2013. https://www.hse.gov.uk/pubns/books/hsg53.htm (accessed 19/04/2020).
- 14. Wetherell A, Mathers G. Respiratory protection. In: Marrs TC, Maynard RL, Sidell FR, eds. Chemical warfare agents – toxicology and treatment, 2nd edn. Chichester: John Wiley and Sons Ltd, 2007: 157–73. [Google Scholar]
- 15. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorbello M, El‐Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia 2020;75:724‐32. [DOI] [PubMed] [Google Scholar]
- 17. Castle N, Pillay Y, Spencer N. Comparison of six different intubation aids for use while wearing CBRN‐PPE: a manikin study. Resuscitation 2011; 82: 1548–52. [DOI] [PubMed] [Google Scholar]
- 18. Castle N, Pillay Y, Spencer N. Insertion of six different supraglottic airway devices whilst wearing chemical, biological, radiation, nuclear‐personal protective equipment: a manikin study. Anaesthesia 2011; 66: 983–8. [DOI] [PubMed] [Google Scholar]
- 19. Cook TM, Boniface NJ, Seller C, et al. Universal videolaryngoscopy: a structured approach to conversion to videolaryngoscopy for all intubations in an anaesthetic and intensive care department. British Journal of Anaesthesia 2018; 120: 173–80. [DOI] [PubMed] [Google Scholar]
- 20. Hall D, Steel A, Heij R, Eley A, Young P. Videolaryngoscopy increases 'mouth‐to‐mouth' distance compared with direct laryngoscopy. Anaesthesia 2020;75:822‐23. [DOI] [PubMed] [Google Scholar]
- 21. Powell JB, Kim JH, Roberge RJ. Powered air‐purifying respirator use in healthcare: effects on thermal sensations and comfort. Journal of Occupational and Environmental Hygiene 2017; 14: 947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chughtai AA, Seale H, Rawlinson WD, Kunasekaran M, Macintyre CR. Selection and use of respiratory protection by healthcare workers to protect from infectious diseases in hospital settings. Annals of Work Exposures and Health 2020; 64: 368–77. [DOI] [PubMed] [Google Scholar]
- 23. Coyne K, Barker D. Speech intelligibility during respirator wear. In: Racz L, Yamamoto D, Eninger R, eds. Handbook of Respiratory Protection. Boca Raton, FL: Taylor and Francis, 2018: 72–91. [Google Scholar]
- 24. UK Government . The Health and Safety at Work etc Act 1974. Ch37. London: The Stationery Office, 1974. http://www.legislation.gov.uk/ukpga/1974/37/contents (accessed 24/04/2020). [Google Scholar]
- 25. Health and Safety Executive . Management of health and safety at work. Management of Health and Safety at Work Regulations 1999. Approved Code of Practice and guidance L21, 2nd edn. HSE Books, 2000. https://www.hseni.gov.uk/sites/hseni.gov.uk/files/publications/%5Bcurrent‐domain%3Amachine‐name%5D/l21‐management‐of‐health‐and‐safety‐at‐work_0.pdf (accessed 24/04/2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Wearer comfort evaluation form.


