The ongoing severe acute respiratory sickness coronavirus 2 (SARS‐CoV‐2) pandemic has resulted in more than 3,600,000 detected cases of COVID‐19 illness and nearly 260,000 deaths worldwide as of May 6, 2020. Recently, BCG vaccination was shown to correlate with reduced COVID‐19 case fatality rates (preprint: Miller et al, 2020; preprint: Sala & Miyakawa, 2020; https://www.jsatonotes.com/2020/03/if-i-were-north-americaneuropeanaustral.html). The most recent data from publicly available resources also indicate that both COVID‐19 incidence and total deaths are strongly associated with the presence or absence of national mandatory BCG vaccination programs. As seen in Table 1, seven of eight countries with very low numbers of total deaths (< 40 per 1 million population) adopted a mandatory BCG vaccination program using one of a set of 6 separate BCG strains (Table 1). In contrast, COVID‐19 mortality was markedly higher in countries where BCG vaccination is not widely administered or is given only to high‐risk groups. COVID‐19 mortality was also higher in countries where widespread BCG vaccination was discontinued more than 20 years ago and in countries that used the BCG Denmark strain regularly or temporarily. This raises the question of whether BCG vaccination and reduced COVID‐19 mortality are causally related. An additional question is why different BCG strains may be variably associated with mortality.
Subject Categories: Immunology; Microbiology, Virology & Host Pathogen Interaction; S&S: Ethics
Is there more to BCG vaccination and reduced COVID‐19 case fatality rates than a mere correlation? Masayuki Miyasaka comments on this timely but intriguing question and provide some interesting points to consider.

BCG (Mycobacterium bovis Bacillus Calmette‐Guérin) is a live attenuated vaccine for tuberculosis (TB) that is given to infants intradermally shortly after birth. In addition to protecting against TB, BCG vaccination has been shown to exert heterologous immune effects to enhance protection against unrelated pathogens (Hirve et al, 2012). Based on clinical findings and experimental data, BCG is hypothesized to induce sustained changes in the immune system that result in heightened responses to infections at the level of innate and adaptive immunity (Mulder et al, 2019; Netea et al, 2020). In innate immune cells, BCG induces histone modifications and epigenetic reprogramming at the promotor sites of genes encoding inflammatory cytokines such as interleukin (IL)‐1, IL‐6, and tumor necrosis factor (TNF). This process has been termed “trained immunity” (Netea et al, 2020) (Table 1).
Table 1.
COVID‐19 deaths per million population and BCG vaccination
| Country | Death/106 a | Universal BCG vaccination programb | BCG strain usedb |
|---|---|---|---|
| Spain | 540 | 1965–1981 | Denmark |
| Italy | 478 | – | – |
| UK | 419 | 1953–2005 | Denmark |
| France | 381 | 1950–2007 | Denmark |
| Sweden | 274 | 1940–1975 | Denmark |
| USA | 207 | – | – |
| Germany | 82 | 1961–1998 | Pasteur |
| Iran | 75 | + | Denmark |
| Finland | 43 | 1941–2006 | Denmark |
| Turkey | 40 | + | India |
| Norway | 39 | ?–2009 | Denmark |
| Korea | 5 | + | Multiple strains? |
| Australia | 4 | 1950‐mid 1980s | Connaught |
| Japan | 4 | + | Japan |
| China | 3 | + | Russia/Bulgaria |
| Iraq | 2 | + | Japan |
| Taiwan | 0.3 | + | Japan |
Obtained from Worldometer (https://www.worldometers.info/coronavirus/).
Obtained from Ritz and Curtis (2009) and Zwerling et al (2011).
The currently used BCG vaccine was initially produced at the Pasteur Institute, Paris, in 1921. The original vaccine strain was subsequently distributed to different laboratories worldwide and maintained by serial passage in each country. As shown in Fig 1, these strains can be classified as “early strains” and “late strains” depending on the timing of distribution. Notably, BCG strains that appear to be associated with lower COVID‐19 mortality (e.g., BCG Japan and BCG Russia) are both early strains, whereas BCG Denmark, which seems to induce less protection against COVID‐19, is a late strain.
Figure 1. History and genetics of BCG vaccine strains.
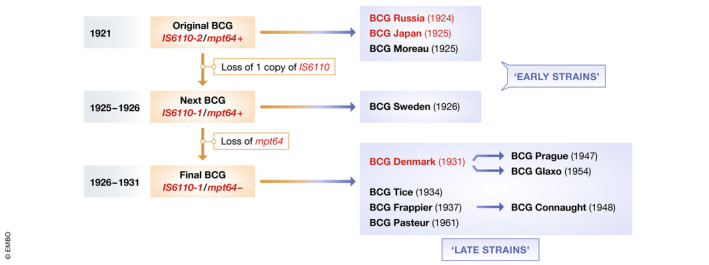
The original BCG strain, first produced in 1921 at the Pasteur Institute, was distributed globally and subsequently maintained by serial passage in each country. Figure modified from Behr and Small (1999).
While one study indicated that early and late BCG strains have comparable abilities to induce delayed‐type immune responses against tuberculin (Table 2; Ladefoged et al, 1976), subsequent studies demonstrated that these strains differ genetically and phenotypically. First, a DNA fingerprinting study demonstrated that late strains such as BCG Denmark have evolved from early strains through genetic mutations (Behr & Small, 1999; partially depicted in Fig 1). Another study indicated that, probably because of these mutations, late strains have lost expression of several membrane proteins including MPB64, MPB70, and MPB83. Moreover, cell wall‐associated lipids including methoxy mycolate are absent in late strains, while phthiocerol dimycocerosates (PDIMs) and phenolic glycolipids (PGLs) are retained (Table 3; Chen et al, 2007; Liu et al, 2009). Another study showed that the early BCG Japan and BCG Russia strains both have much higher bacterial counts than the late strains (Table 4; WHO Technical Report, 1979), raising the interesting possibility that early strains may be richer than late strains in substance(s) that can stimulate immune responses associated with “trained immunity”.
Table 2.
Tuberculin sensitization abilities of different BCG strains in children
| BCG strains | Score |
|---|---|
| Japan | 5 |
| Pasteur | 4 |
| Denmark | 4 |
| Glaxo | 2 |
| Russia | 4 |
| Moreau | 5 |
Data derived from Ladefoged et al (1976).
Table 3.
Cell membrane composition of different BCG strains
| BCG strains | MPB64 | MPB70/83 | Methoxy mycolate | PDIMs/PGLs |
|---|---|---|---|---|
| Russia | + | +++ | + | + |
| Japan | + | +++ | + | − |
| Moreau | + | +++ | + | − |
| Sweden | + | +++ | + | + |
| Denmark | − | + | − | + |
| Glaxo | − | + | − | − |
| Frappier | − | + | − | + |
| Pasteur | − | + | − | + |
Table 4.
Bacterial counts in different BCG strains
| BCG strains (country) | Viable bacterial counts (106/ml) |
|---|---|
| Japan (Japan) | 20–50 |
| Russia (Russia) | 10–30 |
| Glaxo (UK) | 8–26 |
| Connaught (Australia) | 7–15 |
| Moreau (Brazil) | 2–10 |
| Pasteur (France) | 1–10 |
| Pasteur (Netherlands) | 1–10 |
| Denmark (Denmark) | 3–7 |
| Denmark (Germany) | 1–3 |
Data derived from WHO Technical Report Series 638 (1979).
Two studies evaluated BCG Japan and BCG Denmark for their ability to induce cytokine secretion in peripheral blood lymphocytes. One of these, conducted in Africa, showed that BCG Japan induced more robust proliferation of CD4+ and CD8+ T cells, higher secretion of Th1 cytokines (interferon‐γ, TNF‐α, and IL‐2) and lower secretion of Th2 cytokines (IL‐4) compared with BCG Denmark (Davids et al, 2006). Another study conducted in Mexico showed that BCG Japan induced higher levels of IL‐1α, IL‐1β, IL‐6, and IL‐24 in peripheral blood mononuclear cells obtained from vaccinated children compared with BCG Denmark (Wu et al, 2007). These results suggest that BCG Japan is more efficient than BCG Denmark in inducing the production of multiple types of inflammatory cytokines.
While the exact mechanism of action remains unclear, these results collectively raise the interesting possibility that specific BCG strains such as BCG Japan may effectively induce immunity not only against M. tuberculosis but also unrelated pathogens. Thus, one could hypothesize that a particular BCG strain might preferentially act on the immune system in a manner that significantly reduces the morbidity/mortality associated with certain viral infections. However, data from both Finland and Australia seemingly contradict the hypothesis that early BCG strains confer resistance to COVID‐19 morbidity. These countries ceased their universal BCG vaccination programs some years ago (2006 in Finland and mid‐1980s in Australia), yet they show a low mortality of COVID‐19 per 1 million population, compared with countries with current mandatory BCG vaccination (Table 1). Thus, BCG vaccination—if it does contribute to lower COVID‐19 mortality—is clearly not the only factor. Two relevant traits shared by Finland and Australia are their excellent medical care systems and low population densities, and the latter of which could make social distancing measures more effective than in population‐dense countries.
Finally, all the abovementioned studies are observational, and no causal relationship has been substantiated yet between BCG vaccination and reduced numbers of severe and/or fatal COVID‐19 cases. While some countries have begun clinical studies to determine whether BCG vaccination can protect healthcare workers from SARS‐CoV‐2 (not yet recruiting and recruiting clinical trials, with a total of over 10,000 participants—NCT04369794, NCT04350931, NCT04362124, NCT04348370, NCT04327206, NCT04347876, NCT04328441—ClinicalTrials.gov), small‐scale clinical trials with limited numbers of participants are unlikely to provide a clear‐cut answer. This is because < 10 of 1,000 individuals are expected to become infected with SARS‐CoV‐2 even in endemic countries such as Spain, Italy, and France (with incidence of 5,285, 3,485, and 2,584 cases per million population, respectively, as of March 4, 2020). Therefore, such studies are unlikely to document a definitive effect of BCG vaccination. Human challenge studies are another possibility, but ethical issues would prohibit many of these. Instead, given that ferrets are susceptible to SARS‐CoV‐2 infection (Kim et al, 2020), experimental verification of this hypothesis using animal models is warranted and feasible.
Conflict of interest
The author declares that he has no conflict of interest.
Acknowledgement
I would like to thank Jun Sato (Brisbane, Australia) for helpful discussions and Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
References
- Behr MA, Small PM (1999) A historical and molecular phylogeny of BCG strains. Vaccine 17: 915915 [DOI] [PubMed] [Google Scholar]
- Chen JM, Islam ST, Ren H, Liu J (2007) Differential productions of lipid virulence factors among BCG vaccine strains and implications on BCG safety. Vaccine 25: 8114–8122 [DOI] [PubMed] [Google Scholar]
- Davids V, Hanekom WA, Mansoor N, Gamieldien H, Sebastian JG, Hawkridge A, Hussey GD, Hughes EJ, Soler J, Murray RA et al (2006) The effect of Bacille Calmette‐Guérin vaccine strain and route of administration on induced immune responses in vaccinated infants. J Infect Dis 193: 531–536 [DOI] [PubMed] [Google Scholar]
- Hirve S, Bavdekar A, Juvekar S, Benn CS, Nielsen J, Araby P (2012) Non‐specific and sex‐differential effects of vaccinations on child survival in rural western India. Vaccine 30: 7300–7308 [DOI] [PubMed] [Google Scholar]
- Kim Y‐I, Kim S‐G, Kim S‐M, Kim E‐H, Park S‐J, Yu K‐M, Chang J‐H, Kim EJ, Lee S, Casel MAB et al (2020) Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host Microbe 27: S1931‐3128(20)30187‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladefoged A, Bunch‐Christensen K, Guld J (1976) Tuberculin sensitivity in guinea‐pigs after vaccination with varying doses of BCG of 12 different strains. Bull World Health Organ 53: 435–443 [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tran V, Leung AS, Alexander DC, Zhu B (2009) BCG vaccines. Their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccines 5: 70–78 [DOI] [PubMed] [Google Scholar]
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH (2020) Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID‐19: an epidemiological study. medRxiv 10.1101/2020.03.24.20042937 [PREPRINT] [DOI]
- Mulder RJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG (2019) Therapeutic targeting of trained immunity. Nat Rev Drug Discov 18: 553–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Domínguez‐Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM et al (2020) Defining trained immunity and its role in health and disease. Nat Rev Immunol 4: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz N, Curtis N (2009) Mapping the global use of different BCG vaccine strains. Tuberculosis 89: 248–251 [DOI] [PubMed] [Google Scholar]
- Sala G, Miyakawa T (2020) Association of BCG vaccination policy with prevalence and mortality of COVID‐19. medRxiv 10.1101/2020.03.30.20048165 [PREPRINT] [DOI]
- Wu B, Huang C, Garcia L, Ponce de Leon A, Osornio JS, Bobadilla‐del‐Valle M, Ferreira L, Canizales S, Small P, Kato‐Maeda M et al (2007) Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette‐Guérin . Infect Immun 75: 3658–3664 [DOI] [PMC free article] [PubMed] [Google Scholar]


