Abstract
The coronavirus disease 2019 (COVID-19) pandemic poses special challenges to immunocompromised transplant patients. Given the paucity of proven data in treating COVID-19, management of these patients is difficult, rapidly evolving, and mainly based on anecdotal experience. We report 2 cases of heart transplant (HT) recipients with COVID-19. The first is a 59-year-old female with HT in 2012 who presented on March 20, 2020 with fever, hypoxia, and ground-glass opacities on chest X-ray. She quickly progressed to acute hypoxic respiratory failure and vasoplegic shock. Despite reduction in immunosuppression and treatment with tocilizumab, intravenous immunoglobulin, hydroxychloroquine, lopinavir/ritonavir, and broad-spectrum antibiotics, she ultimately died from multiorgan failure. The second case is a 75-year-old man with HT in 2000 who presented on April 2, 2020 after curbside testing revealed positive COVID-19. Given a milder presentation compared to the first patient, antimetabolite was discontinued and only hydroxychloroquine was started. Because of a lack of clinical improvement several days later, tocilizumab, methylprednisolone, and therapeutic anticoagulation were initiated. The patient clinically improved with decreasing oxygen requirements and was discharged home. These 2 cases highlight the wide range of different presentations of COVID-19 in HT recipients and the rapidity with which the management of these patients is evolving.
KEYWORDS: clinical research/practice, complication: infectious, drug toxicity, heart (allograft) function/dysfunction, heart transplantation/cardiology, immunosuppressant, infection and infectious agents - viral, infectious disease, pharmacology
1. INTRODUCTION
As of April 14, 2020, there are 1 935, 646 confirmed cases of coronavirus disease 2019 (COVID-19) worldwide with 120 914 total deaths, defining COVID-19 as a pandemic.1 The limited literature on COVID-19 in heart transplant (HT) patients thus far suggests that HT might not have a disproportionate effect on infection and severity of disease.2 , 3 However, we know this immunosuppressed population is at higher risk than the general population in contracting both viral and bacterial infections. We report 2 cases of COVID-19 in HT patients.
2. CASE 1
The patient is a 59-year-old African-American female with history of nonischemic cardiomyopathy and left ventricular assist device prior to HT in 2012. Her posttransplant course was complicated by cardiac allograft vasculopathy (CAV, Stanford class II, International Society for Heart and Lung Transplantation 0), diabetes mellitus (DM), hypertension (HTN), and chronic kidney disease (CKD) G3b-4/A3, with no graft dysfunction. Immunosuppression regimen consisted of tacrolimus 6 mg twice daily with goal trough level of 4-6 ng/mL and mycophenolic acid (MPA) 360 mg twice daily. She had no recent hospitalizations, travel history, or sick contacts.
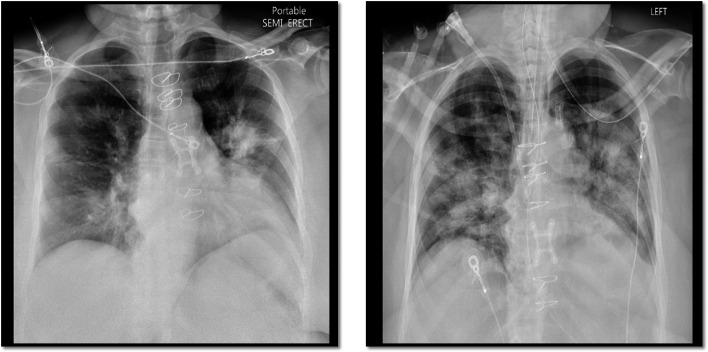
She presented on March 20, 2020 with fever, myalgia, fatigue, diarrhea, productive cough, and shortness of breath for 3 days. Temperature was 38.8C, heart rate 108 bpm, blood pressure 120/90mm Hg, respiratory rate 25, and oxygen saturation 92% on 3L nasal cannula (NC). Notable laboratory values include interleukin (IL)-6 62.7 pg/mL, immunoglobulin G (IgG) 1426 mg/dL, tacrolimus trough 8.5 ng/mL, and creatinine (Cr) 2.6 mg/dL (baseline 1.8-2.0 mg/dL). Additional laboratory values indicating severe disease in COVID-19 are shown in Table 1.4, 5, 6 Chest X-ray showed consolidative opacity in the left upper lobe perihilar region and diffuse bronchial wall thickening with patchy peribronchial ground-glass opacities bilaterally ( Figure 1, left). While awaiting testing for respiratory viruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the patient was started on empiric cefepime, vancomycin, and oseltamavir. Given high suspicion for SARS-CoV-2, MPA was stopped and tacrolimus was held to achieve a goal of 4-6 ng/mL.
TABLE 1.
Case 1
| Parameter and cutoff for adverse outcome | Laboratory values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 | |
| D-Dimer > 1000 ug/mL | 1.29 | 1.19 | 1.22 | 3.88 | 1.06 | 2.1 | 1.68 | 4.78 | 12.65 | 8.27 |
| CPK > 2x ULN U/L | — | — | 86 | 1941 | 1505 | 2714 | 2396 | 1975 | 2038 | 1273 |
| CRP > 100 mg/L | 82 | 110 | 86 | 44 | 42 | 56 | 46 | 46 | 50 | 63 |
| LDH > 245 U/L | 252 | — | 301 | — | — | 778 | 806 | — | 827 | 761 |
| Hs-Tn, ng/L | 55 | 52 | 52 | 51 | 51 | — | — | 37 | 33 | 34 |
| Abs Lymphocyte count < 0.8 10*3/uL | 1.49 | — | 1.36 | 1.52 | — | — | 1.85 | 2.18 | 4.05 | |
| Ferritin > 300 ng/mL | 281 | 889 | 927 | 1417 | — | 3991 | 4342 | 3593 | 3299 | 2732 |
| AST, U/L | 39 | — | 34 | — | — | — | 322 | 265 | 197 | 160 |
| ALT, U/L | 25 | — | 22 | — | — | — | 143 | 129 | 129 | 125 |
Abbreviations: ALT, alanine aminotransferase (8-35 U/L); AST, aspartate aminotransferase (8-37 U/L); CPK, creatine phosphokinase (9-185 U/L); CRP, C-reactive protein (<5 mg/L); Hs-Tn, high sensitivity troponin (<22 ng/L); LDH, lactate dehydrogenase (116-245 U/L); ULN, upper limit of normal.
FIGURE 1.
Chest X-ray of case 1. Left (admission): bilateral diffuse bronchial wall thickening and patchy peribronchial ground-glass opacities as well as consolidative opacity in the left upper lobe perihilar region. Right (day 4): endotracheal tube and worsening of pulmonary opacities
Within hours of presentation on day 0, worsening respiratory failure developed, requiring high flow NC (HFNC). Arterial blood gas (ABG) at time of decompensation was pH 7.3, pCO2 32 mm Hg, and pO2 64 mm Hg on 0.8 FiO2. SARS-CoV-2 resulted positive. Hydroxychloroquine (400 mg twice daily on day 0, followed by 200 mg twice daily for days 1-4) and tocilizumab 400 mg intravenous (1 dose) were initiated. Antibiotic coverage was broadened to include doxycycline. A single dose of intravenous immunoglobulin (IVIG) 10g was given but not continued after a normal IgG level returned. Underlying CKD precluded enrollment into a remdesivir trial (excluded if CrCl < 50 mL/min). As a second option, antiviral therapy with lopinavir/ritonavir 400 mg twice daily for 5 days was added. Consideration was given to drug-drug interactions with tacrolimus.
She initially improved on day 1 and was transitioned to NC. However, she subsequently developed worsening renal failure and acute respiratory distress syndrome (ARDS,Figure 1, right). She was intubated on day 2 and started on continuous veno-venous hemodialysis (CVVHD). ABG at this time showed pH 7.25, pCO2 30mm Hg, pO2 59 mm Hg on FiO2 0.8, and tacrolimus trough was 7.0 ng/mL. She required norepinephrine for vasoplegic shock and antifungal coverage with micafungin 100mg daily was added.
Despite decreasing C-reactive protein, other inflammatory markers including ferritin, d-dimer, and creatinine kinase continued to uptrend (Table 1). High sensitivity troponin (Hs-Tn) remained stable and bedside echocardiogram showed preserved graft function. On days 3 and 4, low tidal volume ventilation was continued with FiO2 0.6 and PEEP 18mmH2O, and on day 5, the patient was prone positioned. Given tacrolimus trough of 6.7 ng/mL, 1 dose of tacrolimus with 60% dose reduction was given, however because of rapidly rising levels, it was held thereafter. On day 7, given rising white blood count and markedly elevated fungitell assay (259 pg/mL, <60 pg/mL), sulfamethoxazole-trimethoprim-DS was started for presumed pneumocystis jirovecii pneumonia. At this point, veno-venous extracorporeal membrane oxygenation (VV-ECMO) was considered for progressive ARDS; however, the difficult decision was made not to offer VV-ECMO due to age, comorbidities, and poor prognosis. Heparin drip was started as the CVVHD line was clotting with rising d-dimer levels. Tobramycin and linezolid were given for worsening leukocytosis. On day 10, the patient’s family was notified of her grave prognosis and care was withdrawn.
3. CASE 2
The patient is a 75-year-old man with history of ischemic cardiomyopathy who underwent HT in 2000. Posttransplant course has been complicated by HTN, DM, CKD, and CAV (Stanford class III, International Society for Heart and Lung Transplantation 0), with no graft dysfunction. Immunosuppression regimen consisted of cyclosporine (CSA) 100mg twice daily (goal trough 75-100 ng/mL) and mycophenolate mofetil (MMF) 250mg twice daily. He had no recent hospitalizations or travel history but lives with his son who is a nurse.
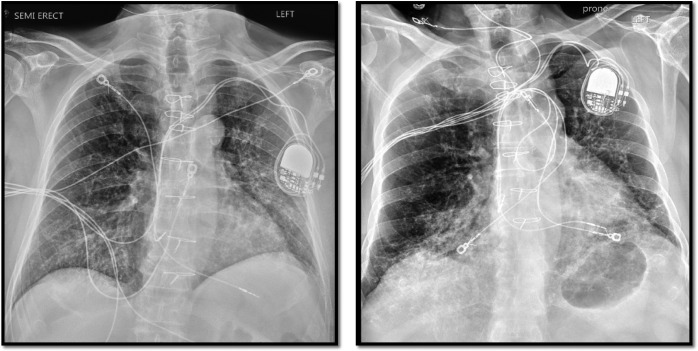
The patient presented on April 2, 2020 after testing positive the day before for SARS-CoV-2 at curbside testing facility. He reported 4 days of low-grade fever, cough, diarrhea, fatigue, and lack of appetite. Upon presentation, temperature was 38.6C, heart rate 87 bpm, blood pressure 111/87 mm Hg, respiratory rate 17, and oxygen saturation 99% on room air. Creatinine was 2.7 mg/dL (baseline 2.0-2.3 mg/dL) and there was no CSA trough on admission because of difficulty with timely blood draws. Other notable laboratory values are summarized in Table 2. Chest X-ray showed chronic lung disease with overlying opacities on the left suspicious for atypical infection ( Figure 2, left). Upon admission, MMF was held and CSA was continued at home dose. Despite a mild presentation, hydroxychloroquine was initiated because of age and comorbidities.
TABLE 2.
Case 2
| Parameter and cutoff for adverse outcome | Laboratory values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | |
| D-Dimer > 1000 ug/mL | 0.71 | — | — | — | 0.68 | 0.69 | 0.87 | 1 | 1.57 |
| CPK > 2x ULN U/L | — | — | — | — | — | — | — | — | — |
| CRP > 100 mg/L | 64 | — | 65 | 87 | 114 | 129 | 217 | 105 | 65 |
| LDH > 245 U/L | 267 | — | — | — | — | 452 | 428 | 431 | 423 |
| Hs-Tn, ng/L | 25 | 26 | — | — | 36 | — | — | — | — |
| Abs Lymphocyte count < 0.8 10*3/uL | — | 2.55 | — | — | — | — | — | — | — |
| Ferritin > 300 ng/mL | 304 | — | — | — | 612 | — | — | — | — |
| AST, U/L | 37 | 36 | 42 | 43 | 38 | 42 | 37 | 33 | 29 |
| ALT, U/L | 17 | 17 | 17 | 14 | 16 | 16 | 18 | 16 | 17 |
Abbreviations: ALT, alanine aminotransferase (8-35 U/L); AST, aspartate aminotransferase (8-37 U/L); CPK, creatine phosphokinase (9-185 U/L); CRP, C-reactive protein (<5 mg/L); Hs-Tn, high sensitivity troponin (<22 ng/L); LDH, lactate dehydrogenase (116-245 U/L); ULN, upper limit of normal.
FIGURE 2.
Chest X-ray of case 2. Left (admission): chronic lung disease with overlying opacities on the left suspicious for atypical infection. Right (day 4): increase in basilar interstitial markings
On days 1-4, the patient remained febrile and nauseated. He was hemodynamically stable and did not require supplemental oxygen. On the evening of the fourth day, he become hypoxic with oxygen saturation of 85% on 2L NC, requiring nonrebreather with improvement to 98%.
Chest X-ray showed increase in basilar interstitial markings (Figure 2, right). Over several hours, he was weaned to 2L NC. Given clinical decompensation, the decision was made on day 5 to give tocilizumab 400mg intravenous (1 dose) and methylprednisolone 40mg intravenous daily for 5 days and to increase lovenox from prophylactic to treatment dosing. He subsequently improved and was discharged on day 8.
4. DISCUSSION
Currently, the mainstay of therapy for SARS-CoV-2 infection remains supportive care and the clinical efficacy for agents outside of supportive care is investigational. The majority of data are extrapolated from antiviral therapy targeting other viruses, such as SARS-COV-1, Middle East respiratory syndrome coronavirus (MERS-CoV), and the Ebola virus.7, 8, 9 It remains unclear how well these data can be generalized to SARS-CoV-2. When considering potential treatment options for COVID-19, it is helpful to differentiate between an initial viral response phase followed by and overlapping with a host inflammatory response phase.10 In transplant patients who are immunosuppressed, a delicate balance is needed to overcome the initial viral response and counteract the subsequent cytokine storm.
Emerging clinical evidence and available in vitro data suggest remdesivir is a promising antiviral agent for treatment of SARS-CoV-2 .11, 12, 13, 14 Remdesivir acts as an RNA chain terminator that displays high genetic barrier to resistance in coronaviruses.15 , 16 Although remdesivir is a substrate for cytochrome P450 enzymes, its metabolism is mediated by hydrolase activity, thus avoiding significant drug interactions with CYP3A4 inhibitors or inducers,16 an important consideration in transplant patients. Among the first 12 patients confirmed by the Centers for Disease Control and Prevention to have COVID-19 in the United States, 3 were treated with remdesivir. All patients are reportedly recovering, but the authors were unable to assess its efficacy or safety.17
In patients not eligible for remdesivir, such as in our patients, hydroxychloroquine with addition of lopinavir/ritonavir (LPV/r) could be considered, acknowledging the limited evidence for these treatments. Chloroquine has been successfully used as a treatment adjunct in China demonstrating clinical and virologic benefit versus a comparison group.18 Hydroxychloroquine has relatively higher in vitro activity against SARS-CoV-2 than chloroquine and is better tolerated.19 Gautret et al demonstrated that use of hydroxychloroquine in COVID-19 patients alone or in combination with azithromycin reduces detection of SARS-CoV-2 RNA in upper respiratory tract secretions. Notably, comparison was done with a nonrandomized control group with no analysis of clinical outcomes, underlining need for further investigation.20 Hydroxychloroquine has a significant side effect profile, and in particular with concurrent use of azithromycin, close monitoring of QTc intervals is warranted. For this reason, doxycycline was used in our patient.
In addition to hydroxychloroquine, our group used lopinavir, a human immunodeficiency virus 1 protease inhibitor administered in combination with ritonavir, a potent CYP3A4 inhibitor that “boosts” lopinavir, inhibiting viral replication. The combination of LPV/r with steroids has been shown to reduce the incidence of ARDS, intubation, and mortality in patients with newly diagnosed SARS-CoV-17 , 21 The compelling mortality difference in SARS-CoV-1 and continued investigation in MERS-CoV led to inclusion of LPV/r in the Chinese SARS-CoV-2 guidelines at a dose of 400mg/100mg twice daily for no more than 10 days.22 Notably, in a recent randomized controlled trial in COVID-19 pneumonia, there was no benefit seen with LPV/r; however, the median time from symptom onset to initiation of therapy was 13 days, and in the SARS-CoV-1 experience, therapy appeared effective if started early.23
It is important to note that protease inhibitors such as ritonavir are potent inhibitors of the metabolism of immunosuppressive drugs including calcineurin inhibitors (CNI) and must be used with caution in transplant patients.24 As CNI are known to affect kidney function, close monitoring is imperative when given with LPV/r, especially in light of observed acute kidney injury in COVID-19 patients and adverse effects on mortality.25 , 26 In our first case, during the course of treatment with LPV/r, daily tacrolimus troughs ranged from 6-8 ng/mL and was 7 ng/mL at the time of initiation of CVVHD on day 2. Following completion of LPV/r, 1 dose of tacrolimus was given at 60% dose reduction. Tacrolimus levels then increased, ranging from 11-13 ng/mL with a peak of 19 ng/mL. Notably, tacrolimus levels peaked after clinical decompensation and development of refractory ARDS, thus it is unclear if supra-therapeutic levels contributed to further deterioration.
Although it is a common practice to withhold or reduce immunosuppression in transplant patients during an acute infection, it must be noted that doing so is not benign and warrants close monitoring of graft function. In our first patient, bedside echocardiography showed normal graft function and stable Hs-Tn levels (Table 1) despite worsening vasoplegic shock. Furthermore, daily tacrolimus troughs never decreased below 6ng/mL. Interestingly there is now a hypothesis that immunosuppression could potentially be protective. Antonio and Silvia recently suggested that CNI T cell inhibition could be protective against the partially T cell–mediated detrimental immune system overactivation and could diminish acute lung injury observed in COVID-19.27, 28, 29 It is interesting to note that in the 2 cases reported in China and our patients, all were on relatively low doses of baseline immunosuppression, potentially supporting this hypothesis.3 Much more data are needed to confirm or dismiss this hypothesis.
Aside from the potential benefit of immunosuppression in the well-described cytokine storm of COVID-19, anti-inflammatory treatment options are also available. We used tocilizumab in both of our cases to counteract the hyperinflammatory state and cytokine storm associated with increased mortality in COVID-19 patients in China.30 Tocilizumab is a humanized monoclonal antibody that inhibits IL-6 receptors. IL-6 is one of the main drivers of the immunologic response in patients with cytokine-release syndrome. Immunotherapy with tocilizumab is part of the Chinese COVID-19 Diagnosis and Treatment Guide (400 mg IV once, option to repeat at 12 hours for max of 800 mg).22 A Chinese case series of 21 patients treated with tocilizumab reported rapid resolution of fever and C-reactive protein, decreased oxygen requirements, and resolution of lung opacities without adverse events.31 Optimal timing of tocilizumab administration is not yet defined.
Similar to other severe respiratory tract infections, there is significant controversy surrounding the role of corticosteroids for management of severe pneumonia due to coronaviruses. The potential benefit of steroids, which is to blunt the inflammatory cascade seen in severe disease, needs to be carefully weighed against the concerns for secondary infections and adverse events of corticosteroid therapy. In MERS-CoV, corticosteroids was associated with delayed time to viral clearance.9 However, recent evidence in SARS-CoV-2 suggested a decrease in mortality in patients with ARDS with use of corticosteroids. 32 Thus, steroids must be considered carefully but could be helpful in the postviremic hyperinflammatory phase.10 A consensus statement from the Chinese Thoracic Society recommends ≤ 0.5-1 mg/kg/day methylprednisolone for ≤ 7 days in select patients, after careful consideration of risks.33 In contrast to a reported COVID-19 HT case from China with a milder course, we opted to not use steroids in our first patient case given long-standing immunosuppression and fear of delayed viral clearance.34 We revised this strategy in the management of our second patient by initiating methylprednisolone 0.5 mg/kg/day as recommended by the Chinese Thoracic Society. Following treatment with tocilizumab and steroids, oxygen requirements decreased.
A phenomenon observed in COVID-19 patients is high incidence of thrombotic events suggesting a disease-specific coagulopathic state. In severe cases, use of anticoagulation with heparin has been shown to decrease mortality.4 , 35 , 36 The evidence for anticoagulation has accumulated following the death of our first patient, who received prophylactic dosing of heparin. For our second patient, we opted for empiric therapeutic anticoagulation following worsening hypoxia and rising d-dimer levels. We cannot rule out the possibility of pulmonary embolism (PE) as a contributor to our first patient’s decompensation; however, Hs-Tn levels remained stable at a low level, decreasing suspicion for a hemodynamically significant PE. Our second patient improved significantly following treatment with steroids, tocilizumab, and therapeutic anticoagulation and was discharged home.
The first patient had a severe presentation ultimately leading to death, and the second patient had a milder presentation and was discharged home. It is interesting to compare our experience with the first reported cases of COVID-19 in HT patients in China in which both patients had good outcomes.3 The first Chinese patient described was similarly far from transplant like our patient, and immunosuppression level was comparable. His course was similar to our second case, with mild symptoms for several days at home, followed by more severe disease with hypoxia, requiring hospitalization. The second HT patient from China had a milder course and was managed as an outpatient. Interestingly, he was transplanted in 2017, but with a similar level of immunosuppression to the first Chinese patient and our patients.
Though more data are needed, the Chinese experience with 87 HT recipients indicates that this special population does not have a higher risk of infection compared to the general population,2 as long as appropriate prevention measures are taken. However, aside from susceptibility, in the presence of comorbidities such as DM, HTN, and CKD as commonly seen in HT recipients and our cases, the risk for adverse COVID-19 outcomes might be substantially increased. 37 , 38 In light of HTN as a risk factor, it is notable that renin-angiotensin-aldosterone system (RAAS) inhibitors have the potential to upregulate angiotensin-converting enzyme 2, the entry receptor for SARS-CoV-2.39 Despite theoretical concerns regarding the effect of RAAS inhibitors on angiotensin-converting enzyme 2, continuation of therapy is recommended in stable patients and there is now evidence for a potentially beneficial effect of RAAS inhibitors in hospitalized patients with COVID-19.40 , 41 In our first patient, losartan was stopped at time of admission given acute kidney injury. The second patient was treated with an alternate class of antihypertensives.
5. CONCLUSION
The appropriate management of SARS-CoV-2 is a rapidly evolving therapeutic challenge and optimal strategy remains unknown, particularly for the critically ill and special populations. It is prudent to quickly initiate supportive therapy with consideration of additional measures for high-risk populations. Importantly, this strategy is not without risk and needs to be weighed against potential adverse events, which remain poorly defined. Another pressing issue is impending drug shortages with increases in use of these agents and disruptions in supply chain. Caution should be applied, as available clinical data are largely uncontrolled, not peer reviewed, or still unpublished.
It is of utmost interest to focus on improving our understanding of the pathophysiology and treatment of COVID-19. Thus, we believe it is essential for clinicians to report their experiences treating COVID-19 in transplant recipients so we may further optimize treatment options.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren Z-L, Hu R, Wang ZW, et al. Epidemiological and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant. 2020;39(5):412–417. doi: 10.1016/j.healun.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant;20(5):533-534;39(5):496-497. [DOI] [PMC free article] [PubMed]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.@HarvardPulm. “Mass General Hospital #COVID19 #CriticalCare Treatment Guide” 2020.
- 6.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;0(0) doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 7.Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 8.Ratia K, Pegan S, Takayama J, et al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc Natl Acad Sci USA. 2008;105(42):16119–16124. doi: 10.1073/pnas.0805240105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a Pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem. 2017;60(5):1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 17.Kujawski SA, Wong KK, Collins JP, et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv. 2020:2020.2003.2009.20032896.
- 18.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Retracted]
- 21.Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Health National Commission (NHC) of the People’s Republic of China. The diagnosis and treatment guide of COVID-19 pneumonia caused by new coronavirus infection 7th Edition pM.
- 23.Cao B, Wang Y, Wen D, et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe covid-19. New Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain AB, Venkataramanan R, Eghtesad B, et al. Effect of coadministered lopinavir and ritonavir (Kaletra) on tacrolimus blood concentration in liver transplantation patients. Liver Transplant. 2003;9(9):954–960. doi: 10.1053/jlts.2003.50171. [DOI] [PubMed] [Google Scholar]
- 25.Bottiger Y, Brattstrom C, Tyden G, Sawe J, Groth CG. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br J Clin Pharmacol. 1999;48(3):445–448. doi: 10.1046/j.1365-2125.1999.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonio R, Silvia M. Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis [published online ahead of print April 3, 2020]. Am J Transplant. 10.1111/ajt.15905 [DOI] [PMC free article] [PubMed]
- 29.Aslam S, Mehra MR. COVID-19: yet another coronavirus challenge in transplantation. J Heart Lung Transplant. 2020;39(5):408–409. doi: 10.1016/j.healun.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;202005615. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed]
- 32.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020; 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 33.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020. [Epub ahead of print]. 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed]
- 39.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. New Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19 [published online ahead of print 2020]. Circ Res. 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.