To the Editor,
We read with interest the study by Luo et al 1 that evaluates the efficacy of tocilizumab (antibody against interleukin‐6 [IL‐6]) in patients with coronavirus disease 2019 (COVID‐19). The authors shared an encouraging experience of utilizing this medication, particularly in patients at risk of developing chemokine storm secondary to COVID‐19. 1 IL‐6, a chemokine, is an important biomarker of inflammation and has been shown in studies as an important predictor of severe COVID‐19. 2 , 3 IL‐6 is responsible for elevation of acute phase reactants, such as C‐reactive protein, serum amyloid A, fibrinogen, and hepcidin, and inhibition of albumin synthesis. The dysregulated production of IL‐6 has been attributed to autoimmunity and chronic inflammation. 4 We performed a systematic review and meta‐analysis to compare IL‐6 in severe and nonsevere patients.
An extensive literature search of PubMed/Medline, Embase, Cochrane, and Web of Sciences was conducted on 20 April 2020 to include all published studies. Two independent reviewers (MA and RF) screened and finalized articles, and performed data extraction. Any discrepancy during these steps was resolved through a mutual discussion. Severe COVID‐19 was defined as either respiratory distress (rate ≥ 30/min, oxygen saturation ≤ 93% at rest, and/or PaO2/FiO2 ≤ 300 mm Hg), 5 ICU admission, and/or death. Continuous variables (using mean serum levels and standard deviation) were compared and mean difference (MD) was estimated with 95% confidence interval (CI), and P value less than .05 was considered as statistically significant. DerSimonian Laird method/random effects meta‐analysis using Open Meta Analyst (CEBM, University of Oxford, Oxford, UK) was performed. Meta‐regression was attempted if studies did not give a direct comparison between groups of interest. Coefficient (Q), 95% CI, P value (<.05 was considered statistically significant), and scatter plot were generated for regression analysis.
A total of nine studies with laboratory‐confirmed 1426 patients (mean age: 53.0 ± 6.4 years, females: 46.6%) were included. All studies originated from China, and study duration ranged from 1 January to 28 February 2020. A comparison of mean serum IL‐6 for severe COVID‐19 and nonsevere COVID‐19 was performed in seven studies. The mean serum IL‐6 was 56.8 (41.4‐72.3 pg/mL) and 17.3 pg/mL (13.5‐21.1 pg/mL) for severe and nonsevere COVID‐19 group, respectively. This was statistically significant (MD: 38.6 pg/mL, 95% CI: 24.3‐52.9 pg/mL; P < .001, I 2 = 98.5%) (Figure 1A). The results of leave‐one‐out meta‐analysis, with point estimate (MD) ranging between 31.9 and 43.9 pg/mL, were consistent. A subgroup analysis of studies using a strict definition of respiratory distress for severe COVID‐19 also showed consistent results (MD: 26.5 pg/mL, 95% CI: 17.2‐35.8 pg/mL; P < .001, I 2 = 95.7%) (Figure 1B).
Figure 1.
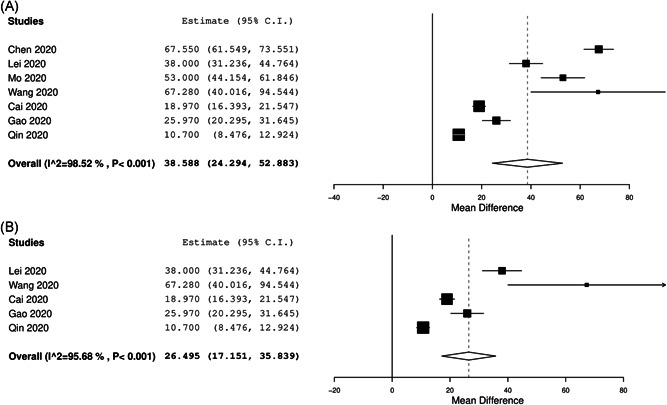
A forest plot comparing mean difference between severe and nonsevere coronavirus disease 2019 patients (A). B, Overall studies using respiratory distress as definition for severity. CI, confidence interval
A total of five studies reported data on overall mortality and serum IL‐6 in COVID‐19 patients. The pooled prevalence of mortality across these studies was 2.9% (95% CI: 1.8%‐4.0%). Meta‐regression demonstrated that increasing mean IL‐6 on admission was associated with an increased likelihood of mortality (Q: 0.01, 95% CI: 0.01‐0.03; P = .03) (Figure 2).
Figure 2.
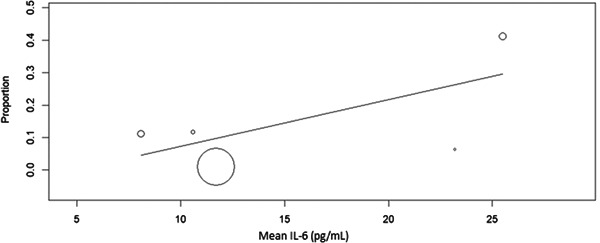
A scatter plot demonstrating the association of serum interleukin‐6 (IL‐6) and mortality
Several limitations exist within our meta‐analysis; the most important is the observational nature of studies and significant heterogeneity in study results. This can be explained on the basis of different patient population, difference in underlying comorbidities, variation in follow‐up, and the presence of coinfection. Despite the limitations, our results remained consistent across both sensitivity and subgroup analysis, demonstrating the importance of obtaining serum IL‐6. Although the studies did not stratify data based on mortality, we were able to demonstrate the association of elevated serum IL‐6 and increased mortality rates using meta‐regression. Another important limitation to note is the variability in laboratory assay when assessing serum IL‐6, as local laboratories have different normal ranges based on local data. 6 This confounding variable can somewhat undermine our results and our data should be interpreted as such, keeping in mind this important limitation.
Based on our analysis, we suggest a cut‐off of more than 55 pg/mL for identifying patients at high risk of severe COVID‐19. Only one study directly compared mean serum IL‐6 level for survivors and nonsurvivors. 5 Based on this, a cut‐off of more than 80 pg/mL can be used for identifying patients at high risk of mortality.
The elevation of IL‐6 has been previously demonstrated in inflammatory state for multiple conditions. 3 The pathophysiological hallmark of COVID‐19 is the severe inflammation and chemokine storm, which explains the elevation of IL‐6. 7 , 8 The importance of identifying this elevated biomarker also lies in the potential use of antibody against IL‐6 such as tociluzumab, which is currently undergoing a clinical trial. 9 Tociluzumab has previously shown efficacy against autoimmune and inflammatory conditions such as rheumatoid arthritis, systemic juvenile idiopathic arthritis,Castleman's disease, neuromyelitis optica, giant cell arteritis, and cytokine release syndrome. 10 , 11
Based on our results, IL‐6 is an important marker of inflammation and can guide the clinicians in recognizing patients with severe COVID‐19 early in the disease course. Furthermore, researchers should develop a scoring system including IL‐6 to assist clinicians in early recognition of patients at risk for developing severe disease.
REFERENCES
- 1. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience [published online ahead of print April 6, 2020]. J Med Virol. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China [published online ahead of print April 2, 2020]. Allergy. 10.1111/all.14309 [DOI] [PubMed] [Google Scholar]
- 4. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wians FH. Clinical laboratory tests: which, why, and what do the results mean? Lab Med. 2009;40:105‐113. [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China [published online ahead of print March 16, 2020]. Clin Infect Dis. 10.1093/cid/ciaa270 [DOI] [Google Scholar]
- 9. ClinicalTrials.gov . Tocilizumab in COVID‐19 pneumonia (TOCIVID‐19). https://clinicaltrials.gov/ct2/show/NCT04317092. Accessed April 1, 2020.
- 10. Oldfield V, Dhillon S, Plosker GL. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs. 2009;69:609‐632. 10.2165/00003495-200969050-00007 [DOI] [PubMed] [Google Scholar]
- 11. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist. 2018;23:943‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]


