To the Editor,
In December 2019, a series of unexplained pneumonia cases appeared which clinical manifestations suggested viral pneumonia. Deep sequencing of lower respiratory tract samples identified a novel coronavirus named the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), and the disease it caused was named coronavirus disease 2019 (COVID‐19). 1 By the end of March 2020, the number of patients with COVID‐19 in China has reached 82 545 and 3314 deaths. A total of 750 890 confirmed cases and 36 405 deaths were registered worldwide. 2 The number of confirmed cases in Wuhan steadily declined from 18 February 2020, 12 days after the first Fangcang shelter hospitals started admitting patients. 3 Unfortunately, there have been some readmission patients due to redetectable positive results of SARS‐CoV‐2 RNA test in Wuhan recently. Among them, we found three readmission patients with IgM negative and positive IgG antibodies during hospitalization. However, there is currently no reported study on readmission COVID‐19 patients with antibodies. Here, we report three readmission patients of COVID‐19 with negative IgM and positive IgG, and describe their diagnosis and treatment process, fever symptoms, computed tomography (CT) images, and the dynamic changes of IgM and IgG antibodies during the first and second admissions.
Three readmission patients with COVID‐19 admitted to Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from 12 February to 1 March 2020. One of them was male and two were female. The ages of the patients was 36, 74, and 34 years old, respectively. These three patients were admitted to Tongji Hospital from 12 February to 1 March 2020. Epidemiological data showed that all three patients had a long history of residence in Wuhan. Case 2 had a medical history of hypertension.The first onset date of these three patients was 19 January to 8 February 2020. All three patients presented with a fever and two of them had a cough. Patients' body temperatures fluctuated within the range of 37.1°C to 38.6°C. The respiratory rate of three patients were 19, 20, and 17 per minute, respectively. The length of the first hospitalization of these three patients was 10, 16, and 10 days, respectively. Diagnosis of patients was confirmed by a positive SARS‐CoV‐2 RNA test. 4 All three patients had a chest CT scan and showed multiple patchy ground‐glass shadows in lungs. The readmission of the three patients was from 10 March to 20 March 2020 due to positivity for the SARS‐CoV‐2 RNA test. During there readmission period, the patient's temperature was normal and there was no special symptom. The respiratory rate of three patients were 20, 18, and 18 per minute, respectively. The time from the first discharge to the second admission was 7, 12, and 9 days, respectively. The length of the second hospitalization of case 1 was more than 17 days while case 2 was 16 days, and case 3 was 11 days.
The SARS‐CoV‐2 RNA test of all three patients was positive before being admitted to hospital. The results of SARS‐CoV‐2 RNA test during the two periods of hospitalization were shown in Table 1. During the first hospitalization, physical examination revealed normal vital signs of three patients with oxygen saturation of 99%, 99%, and 98%, respectively, while the patients were breathing ambient air. Some relevant auxiliary examinations such as blood routine, urine routine, stool routine, coagulation function, liver and renal function, electrolytes, and inflammation indicators were completed and the results were normal. IgM test for respiratory syncytial virus, adenovirus, influenza A and B, parainfluenza, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae were negative. All patients were given treatments such as anti‐infection medicaitons, antiviral medicaitons, Traditional Chinese Medicine therapy, immunomodulatory medicaitons, and oxygen therapy were given in time as well. Furthermore, systematic health education was given to the three patients for building confidence in coping with the disease. The antiviral medications used include Abidol, ribavirin, hydroxychloroquine sulfate, and chloroquine phosphate tablets; antibacterial medicaitons include azithromycin, moxifloxacin, and amoxicillin; Traditional Chinese Medicines include Naoxintong capsule and Lianhua Qingwen capsule; and immunomodulators include thymosin, tocilizumab, human immunoglobulin, and human blood albumin. After the treatment, the symptoms of all three patients were alleviated, and they maintained normal body temperature. The dynamics of chest high‐resolution CT revealed gradual absorption of lung lesions, as shown in Figure 1. Nasopharyngeal swab tests of SARS‐CoV‐2 RNA were performed repeatedly for surveillance, the results were negative for at least two consecutive times (sampling interval ≥ 1 day), which meets the discharge standards. All patients were isolated in hotel for 2 weeks after discharge. All three patients were observed in isolation hotel after the first discharge, and continuously treated with Traditional Chinese Medicine. Although no special discomfort was found, all patient's nasopharyngeal swab tests of SARS‐CoV‐2 RNA were positive during the isolation. All three patients had no fever, only one patient has developed the symptom of cough.
Table 1.
Results of SARS‐CoV‐2 RNA and antibody tests of readmission patients
| Case | Before hospitalization | First hospitalization | Hotel Isolation | Readmission | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First (date) | Second (date) | First | Second | Third (date) | First (date) | First | Second | Third (date) | |||||||||
| (Date) | IgM | IgG | (Date) | IgM | IgG | (Date) | IgM | IgG | (Date) | IgM | IgG | ||||||
| Case 1 | +(2/8) | +(2/24) | −(2/27) | <10 | 89.26 | −(3/2) | <10 | 82.39 | # | +(3/14) | +(3/19) | <10 | 36.28 | +(3/22) | <10 | 41.92 | +(3/23) |
| Case 2 | +(2/3) | # | +(2/13) | <10 | 106.15 | −(2/19) | # | # | −(2/25) | +(3/10) | −(3/10) | <10 | 231.54 | −(3/13) | <10 | 226.66 | −(3/17) |
| Case 3 | +(2/20) | +(2/27) | −(3/2) | 24.87 | 134.60 | −(3/5) | <10 | 162.71 | −(3/8) | +(3/19) | +(3/21) | <10 | 73.39 | −(3/26) | <10 | 78.67 | −(3/28) |
Note: For results of SARS‐CoV‐2 RNA, “+”: positive; “−”: negative; “#”: no test. For results of antibody tests, “>10”: positive; “< 10”: negative; “#”: no test.
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Figure 1.
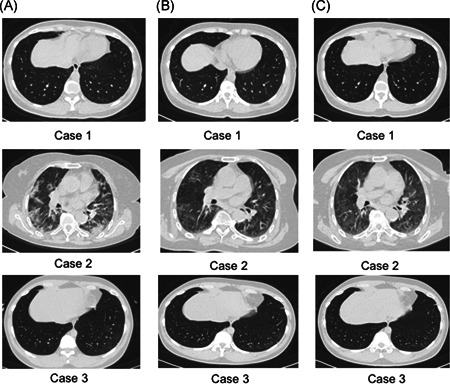
Chest CT images of the three patients. A, Before first admission. B, During the first hospitalization period. C, During the readmission period
After readmission, CT examination showed that the lesions of all three patients were further absorbed compared with first hospitalization period, see Figure 1. Blood routine, urine routine, and stool routine tests, coagulation function, liver and renal function, electrolytes, and inflammation indicators were completed and the results were normal. IgM test for respiratory syncytial virus, adenovirus, influenza A and B, parainfluenza, L. pneumophila, M. pneumoniae, and C. pneumoniae were negative. During the second hospitalization, subcutaneous injection of thymic pentapeptide was used as a treatment for immunity. Hydroxychloroquine sulfate, chloroquine phosphate tablets, and abidol hydrochloride tablets were used for antiviral therapy. Azithromycin and moxifloxacin were used for anti‐infection. Symptomatic treatment of glycyrrhizin diamine enteric‐coated capsules for protecting liver and reducing enzymes. The nasopharyngeal swab test of SARS‐CoV‐2 of three patients were seen in Table 1. The result of case 1 was still positive, and she stayed in hospital for further treatment until now. The results of case 2 and 3 turned negative for two consecutive times. They were discharged and were encouraged to maintain hotel isolation for at least 14 days.
It is reported that detectable and continuous high level of IgM indicated the acute phase of infection of SARS‐CoV‐2. 5 IgG responded later than IgM and persisted at high levels in SARS‐CoV‐2 infected patients, indicating the humoral immune reaction to protect the body against the SARS‐CoV‐2 virus. Details of dynamic changes and results of IgM and IgG antibodies were listed in Table 1. For the first test, IgM was negative in cases 1 and 2 and positive but in low level in case 3, while IgG was positive in all three patients. The results for IgM were negative and positive for IgG when all three patients were discharged. During readmission to hospital, the results were still negative for IgM and positive for IgG antibodies. Compared with the first admission, IgG levels declined in case 1 and 3, while it increased in case 2.
There could be a few possible reasons for the fluctuation of the results of the SARS‐CoV‐2 RNA retests while all three patients were negative for IgM and positive for IgG. First of all, these patients were isolated in a single room after being discharged, they were not exposed to new sources of infection. It is not proved that the patients had contagious virus by detection of specific gene fragments. The detoxification procedure does occur which will cause redetectable positive SARS‐CoV‐2 RNA in recovered patients with COVID‐19. 6 Furthermore, viral RNA detection is almost the only way to confirm the infection of SARS‐CoV‐2 in practice. However, various factors could lead to reduced sensitivity by RNA detection, such as sample type, specimen collection and transport, RNA extraction, detection reagents, and viral load, which proposes serious challenge to disease control. 7 Therefore, even if the RNA test is negative, it cannot be used as a basis for exclusion of COVID‐19, and it needs to be comprehensively judged in combination with actual conditions. Lastly, with the continued passage of SARS‐CoV‐2, it may show a tendency of weakened toxicity and enter into a symbiosis between human and virus. 8 As a result, the recovered patients with COVID‐19 may become silent carriers. Recent studies indicated that the concentration of antibodies in the plasma was independently associated with disease severity in patients with COVID‐19. 9 , 10 However, these patients redetectable positive for SARS‐CoV‐2 RNA displayed high levels of IgG and negative IgM in the plasma during two hospitalization periods. Future studies are needed to investigate humoral immune reaction to the SARS‐CoV‐2 virus.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
ETHICAL APPROVAL
This study was approved by the Ethics Committee of Tongji Hospital (TJ‐IRB20200353). Patient identities were protected via anonymization, and the requirement for informed consent was waived due to the observational nature of the study.
ACKNOWLEDGMENTS
This study was supported by grants from the general program of Health Commission of Hubei Province (WJ2019M137) and HUST COVID‐19 Rapid Response Call (2020kfyXGYJ015).
Funding Information General program of Health Commission of Hubei Province, Grant/Award Number: WJ2019M137; HUST COVID‐19 Rapid Response Call, Grant/Award Number: 020kfyXGYJ015
Contributor Information
Qian Chen, Email: cherrycq81@163.com.
Tao Wang, Email: wt7636@126.com.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Novel coronavirus(2019‐nCoV) situation report‐71. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed March 31, 2020.
- 3. Chen SM, Zhang ZJ, Yang JT, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305‐1314. 10.1016/S0140-6736(20)30744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. General Office of National Health Commission , General Office of National Administration of Traditional Chinese Medicine. Diagnostic and treatment protocol for novel coronavirus pneumonia (Trial version7, revised form). 2020. http://www.gov.cn/zhengce/zhengceku/2020-03/04/content_5486705.htm. Accessed March 4, 2020.
- 5. Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS‐CoV‐2: the first report [published online ahead of print March 21, 2020]. J Infect. 2020. 10.1016/j.jinf.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen DB, Xua WX, Lei ZY, et al. Recurrence of positive SARS‐CoV‐2 RNA in COVID‐19: a case report. Int J Infect Dis. 2020;93:297‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019 [published online ahead of print March 28, 2020]. Clin Infect Dis. 2020:ciaa344. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An JH, Liao XJ, Xiao TY, et al. Clinical characteristics of the recovered COVID‐19 patients with re‐detectable positive RNA test [published online ahead of print March 30, 2020]. medRxiv. 2020. 10.1101/2020.03.26.20044222 [DOI] [Google Scholar]


