The prevalence of cancer in patients with the novel coronavirus disease 2019 (COVID‐19), caused by the SARS‐CoV‐2 infection, is uncertain. In a pooled meta‐analysis including 11 retrospective studies, the prevalence of cancer was 2%. 1 Patients with cancer and COVID‐19 have been reported to have a higher risk of suffering severe events (intensive care unit admission, invasive ventilation, death) in a retrospective study including 18 patients with cancer. 2 One study reported 13 COVID‐19 cases in a cohort of 128 hospitalised patients with haematological cancers, with no significant differences in baseline co‐variates between patients developing or not developing COVID‐19. 3 In this series, cases with concurrent haematologic neoplasm and COVID‐19 had a more severe manifestation of the disease, including acute respiratory distress syndrome (ARDS), and a higher death toll compared to 11 hospitalised healthcare providers with COVID‐19 (8/13 vs. 0/11). In an attempt to reduce the risk of infection among patients with haematologic cancer, some recommendations have been published. 4
A retrospective single‐centre analysis of patients with haematological malignancies who developed COVID‐19 during follow‐up was conducted. Only hospitalised patients or those who underwent hospitalisation due to SARS‐CoV‐2 infection were included. Clinical data were obtained from electronic medical records. Cases were defined as COVID‐19 by clinical, laboratory and imaging (X‐ray) criteria. From March 9 to April 17, 34 cases were identified. Real‐time polymerase chain reaction (RT‐PCR) of nasopharyngeal swab was performed in all cases, with 27 patients testing positive. The seven remaining patients who tested negative were included based on highly clinical and radiological suspicion. Patients were divided into survivors and non‐survivors. Cytometric Bead Array was used to detect the protein levels of interleukin 6 (IL‐6) of patients considered for tocilizumab treatment. The criteria for initiating tocilizumab were ARDS development and high IL‐6 level (over 40 pg/ml, or lower if there was previous treatment with corticosteroids). Means between groups were compared using independent group t‐test and Mann–Whitney test for normally and abnormally distributed data, respectively. Proportions for categorical variables were compared by chi‐square test or Fisher's test when appropriate. Survival outcomes were analysed according to Kaplan–Meier estimator. Overall survival (OS) was defined as the time from COVID‐19 diagnosis to death by any cause or to the last follow‐up date. Cox proportional‐hazards models were constructed based on univariate and multivariate analysis results, and variables for multivariate models were chosen based on clinical relevance.
A complete description of the cohort is presented in Table SI. Table I summarises and compares co‐variates between COVID‐19 survivors (n = 23) and non‐survivors (n = 11). Eight patients received weight‐based dosing of tocilizumab during follow‐up, 2/8 double‐dose and 6/8 single‐dose. IL‐6 levels before and after tocilizumab administration, as well as the outcome of these patients is presented in Figure S1. IL‐6 was tested in six other patients, only 1/6 – in the survivors’ group – showed high levels (150 pg/ml), but was ARDS free. Median follow‐up of the global cohort was 26 days, and OS after this time was 67% (Figure S2). At the end of follow‐up, five patients were still hospitalised. OS is shown according to haematologic malignancy diagnosis before COVID‐19 (Figure 1A). Patients were stratified into four groups: (i) lymphoproliferative disorders (LPDs), including non‐Hodgkin lymphoma, Hodgkin lymphoma and chronic lymphocytic leukaemia; (ii) plasma cell dyscrasias, (iii) acute leukaemias and (iv) myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). OS comparison was also performed according to haematologic treatment purpose at the moment of illness onset based on three groups: (i) induction to remission or salvage therapy, (ii) long‐term treatment (maintenance of remission or stabilization of the disease) and (iii) no treatment (watch and wait or remission with no treatment) (Figure 1B). Five cases (1 AML, 3 MDSs and 1 MPN) were on life support at the time of COVID‐19 diagnosis and were excluded from the survival analysis according to haematologic treatment purpose. Figure 1C shows OS depending on Quick SOFA (qSOFA) Score, CURB‐65 Pneumonia Severity Score, and ECOG Performance Status (ECOG PS) at first hospital admission or at COVID‐19 onset. OS according to the development of moderate or severe ARDS during the hospitalisation period is presented in Figure 1D. Univariate Cox proportional‐hazards models are described in Table SII. Being over 80 years of age, qSOFA on admission/onset ≥2, ECOG PS on admission/onset ≥2, and ARDS development were statistically significantly associated with higher risk of mortality, while treatment with hydroxycloroquine or azythromycin was associated with lower risk of mortality. Multivariate analysis of OS was performed using six variables (Table SIII): being over 80 years of age, haematologic malignancy status at the time of COVID‐19, ECOG PS on admission/onset, treatment with hydroxycloroquine, treatment with azythromycin and ARDS development during follow‐up. The haematologic status, ECOG PS and ARDS were independent variables associated with OS.
Table I.
Characteristics of COVID‐19 patients: total cohort, survivors and non‐survivors.
| Characteristics | Total (n = 34) | Survivors (n = 23) | Non‐survivors (n = 11) | P values |
|---|---|---|---|---|
| Demographics and haematologic malignancy | ||||
| Age, years | ||||
| Median (range) | 72·5 (35–94) | 71 (35–93) | 83 (53–94) | 0·068 |
| ≥80, n (%) | 10 (29·4) | 4 (17·4) | 6 (54·6) | 0·045 |
| Gender, n (%) | ||||
| Male | 19 (55·9) | 14 (60·9) | 5 (45·4) | 0·475 |
| Female | 15 (44·1) | 9 (39·1) | 6 (54·6) | |
| Haematologic malignancy diagnosis, n (%) | ||||
| Acute leukaemia | 7 (20·6) | 3 (13) | 4 (36·4) | 0·005 |
| Myelodisplastic syndrome | 3 (8·8) | 0 (0) | 3 (27·3) | |
| Myeloproliferative neoplasm | 5 (14·7) | 2 (8·7) | 3 (27·3) | |
| Chronic lymphocytic leukaemia | 6 (17·6) | 6 (26·1) | 0 (0) | |
| Non‐Hodgkin lymphoma | 5 (14·7) | 5 (21·7) | 0 (0) | |
| Hodgkin lymphoma | 1 (2·9) | 1 (4·3) | 0 (0) | |
| Plasma cell dyscrasia | 7 (20·6) | 6 (26·1) | 1 (9·1) | |
| Stem cell transplant receptor, n (%) | ||||
| Allogeneic | 1/3 (33·3) | 1/3 (33·3) | 0 (0) | 0·535 |
| Autologous | 2/3 (66·7) | 2/3 (66·7) | 0 (0) | |
| Haematologic malignancy status, n (%) | ||||
| Initial diagnosis or first‐line treatment | 7 (20·6) | 4 (17·4) | 3 (27·3) | 0·021 |
| Relapsed or refractory | 7 (20·6) | 4 (17·4) | 3 (27·3) | |
| Stable, no remission | 8 (23·5) | 3 (13) | 5 (45·4) | |
| Remission without treatment* or watch & wait | 12 (35·3) | 12 (52·2) | 0 (0) | |
| Most recent treatment for haematologic malignancy, n (%) | ||||
| Treatment at the time of COVID‐19 diagnosis | 16/23 (69·6) | 10/16 (62·5) | 6/7 (85·7) | 0·343 |
| Within 6 months prior to COVID‐19 | 3/23 (13) | 2/16 (12·5) | 1/7 (14·3) | |
| >6 months prior to COVID‐19 | 4/23 (17·4) | 4/16 (25) | 0/7 (0) | |
| Clinical and laboratory findings at illness onset or on admission | ||||
| Time from illness onset to admission, days | ||||
| Median (range) | 5 (0–27) | 6 (0–27) | 3 (0–13) | 0·072 |
| Fever (temperature ≥37·5°C) | ||||
| n (%) | 31 (91·2) | 22 (95·7) | 9 (81·8) | 0·239 |
| Symptoms, n (%) | ||||
| Dyspnea | 19/31 (61·3) | 12/21 (57·1) | 7/10 (70) | 0·813 |
| Cough | 20/31 (64·5) | 14/21 (66·7) | 6/10 (60) | |
| Sputum | 5/31 (16·1) | 3/21 (14·3) | 2/10 (20) | |
| Fatigue | 14/31 (45·2) | 10/21 (47·7) | 4/10 (40) | |
| Diarrhoea, nausea and/or vomiting | 11/31 (35·5) | 6/21 (28·6) | 5/10 (50) | |
| Headache and/or confusion | 5/31 (16·1) | 5/21 (23·1) | 0/10 (0) | |
| Other | 11/31 (35·5) | 7/21 (33·3) | 4/10 (40) | |
| qSOFA Score, n (%) | ||||
| <2 | 29 (85·3) | 21 (91·3) | 8 (72·7) | 0·3 |
| ≥2 | 5 (14·7) | 2 (8·7) | 3 (27·3) | |
| CURB‐65 Score, n (%) | ||||
| 0–1 | 14 (41·2) | 11 (47·8) | 3 (27·3) | 0·084 |
| 2 | 10 (29·4) | 8 (34·8) | 2 (18·2) | |
| 3–5 | 10 (29·4) | 4 (17·4) | 6 (54·6) | |
| ECOG performance status, n (%) | ||||
| <2 | 23 (67·6) | 19 (82·6) | 4 (36·4) | 0·016 |
| ≥2 | 11 (32·4) | 4 (17·4) | 7 (63·6) | |
| Infiltrate by chest X‐ray, n (%) | ||||
| No | 4 (11·8) | 2 (8·7) | 2 (18·2) | 0·58 |
| Yes | 30 (88·2) | 21 (91·3) | 9 (81·8) | |
| RT‐PCR SARS‐CoV2 from pharyngeal swab, n (%) | ||||
| Negative | 7 (20·6) | 3 (13) | 4 (36·4) | 0·178 |
| Positive | 27 (79·4) | 20 (87) | 7 (63·6) | |
| Lactate dehydrogenase level, U/l | ||||
| Median (range) | 382 (149–1277) | 357 (149–890) | 550 (165–1277) | 0·147 |
| Creatine kinase level, U/l | ||||
| Median (range) | 70·5 (14–652) | 53 (14–652) | 119 (27–391) | 0·313 |
| C‐reactive protein level, mg/l | ||||
| Median (range) | 114 (6–600) | 111 (6–349) | 117 (37–600) | 0·811 |
| Procalcitonin level, ng/ml | ||||
| Median (range) | 0·2 (0·01–32·7) | 0·2 (0·01–32·7) | 1 (0·16–4·5) | 0·013 |
| D‐dimer level, ng/ml | ||||
| Median (range) | 1 (0·4–9·4) | 0·9 (0·4–9·4) | 1·2 (0·6–1·9) | 0·457 |
| Haemoglobin level, g/l | ||||
| Median (range) | 116 (58–167) | 123 (67–153) | 99 (58–167) | 0·05 |
| Platelet count, ×109/l | ||||
| Median (range) | 170 (16·3–921) | 201 (22·3–921) | 50 (16·3–609) | 0·016 |
| Neutrophil count, ×109/l | ||||
| Median (range) | 5·8 (0–64·2) | 4·8 (0–41·1) | 7·4 (0–64·2) | 0·367 |
| Lymphocyte count, ×109/l | ||||
| Median (range) | 1·43 (0–153) | 1·29 (0–153) | 3·6 (0–25·2) | 0·811 |
| COVID‐19 treatment and outcomes | ||||
| Lopinavir‐Ritonavir* | ||||
| n (%) | 18 (52·9) | 13 (56·5) | 5 (45·4) | 0·545 |
| Hydroxycloroquine † | ||||
| n (%) | 29 (85·3) | 22 (95·7) | 7 (63·6) | 0·029 |
| Azythromycin † | ||||
| n (%) | 17 (50) | 15 (65·2) | 2 (18·2) | 0·01 |
| Other antibiotics | ||||
| n (%) | 30 (88·2) | 21 (91·3) | 9 (81·8) | 0·58 |
| Corticosteroids (Methylprednisolone) | ||||
| n (%) | 17 (50) | 11 (47·8) | 6 (54·6) | 0·714 |
| 0·5 or 1 mg per kg per day, n (%) | 8/17 (47) | 5/11 (45·4) | 3/6 (50) | 0·526 |
| High dose bolus (125, 250 or 1000 mg per day), n (%) | 7/17 (41·2) | 4/11 (36·4) | 3/6 (50) | |
| Both 0·5 or 1 mg per kg per day and high dose bolus, n (%) | 2/17 (11·8) | 2/11 (18·2) | 0/6 (0) | |
| Time from illness onset to Corticosteroids (Methylprednisolone), days | ||||
| Median (range) | 11 (2–27) | 13 (2–27) | 9 (3–17) | 0·407 |
| Tocilizumab | ||||
| n (%) | 8 (23·5) | 5 (21·7) | 3 (27·3) | 1·0 |
| Time from illness onset to Tocilizumab, days | ||||
| Median (range) | 12 (5–37) | 12 (10–37) | 8 (5–14) | 0·177 |
| Moderate or severe ARDS | ||||
| n (%) | 17 (50) | 7 (30·4) | 10 (90·9) | 0·001 |
| Time from illness onset to ARDS, days | ||||
| Median (range) | 7 (0–34) | 9 (2–34) | 7 (0–21) | 0·545 |
| Mechanical ventilation | ||||
| n (%) | 4 (11·8) | 0 (0) | 4 (36·4) | 0·007 |
| ICU admission | ||||
| n (%) | 2 (5·9) | 0 (0) | 2 (18·2) | 0·098 |
| Time from illness onset to discharge or death, days | ||||
| Median (range) | 17 (3–32) | 18·5 (6–32) ‡ | 11 (3–31) | 0·288 |
P values refer to comparison between survivors and non‐survivors’ groups. ARDS, acute respiratory distress syndrome; COVID‐19, novel coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group Performance Status; ICU, intensive care unit; qSOFA, Quick SOFA; RT‐PCR, real‐time polymerase chain reaction.
Including two patients in complete remission under maintenance up to 2 years.
At least 3 days of treatment.
Excluding 5/23 patients still hospitalised.
Fig 1.
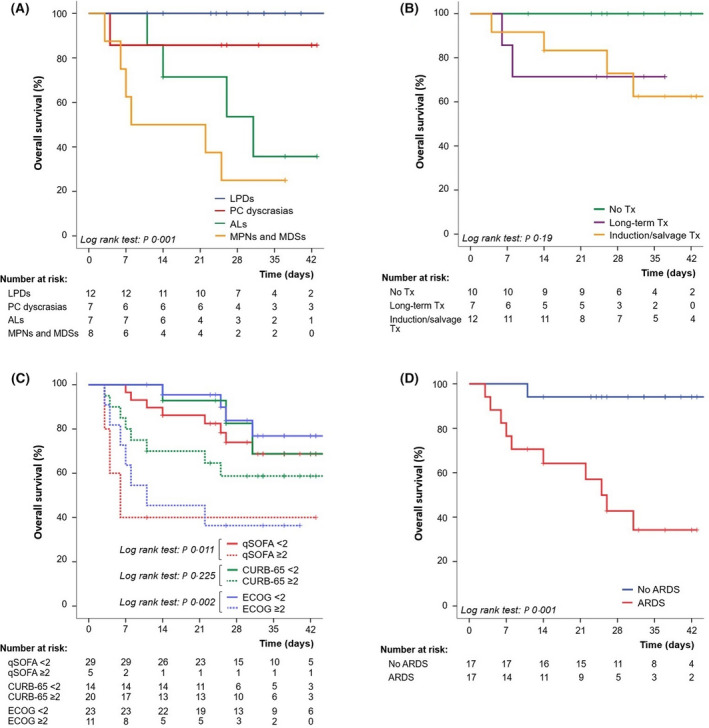
Overall survival according to haematologic malignancy diagnosis previous to SARS‐CoV‐2 infection. (A) Overall survival according to haematologic treatment purpose at the time of illness onset, excluded five cases on life support. (B) Overall survival depending on qSOFA, CURB‐65 and ECOG PS at first hospital admission or at COVID‐19 onset. (C) Overall survival according to the development of moderate or severe ARDS during hospitalisation period.
We report the first series of hospitalised patients with concurrent haematological malignancies and COVID‐19, comparing survivors and non‐survivors. In our series, patients with no active cancer (watch and wait strategy and remission status) presented better outcomes. All patients subjected to symptoms‐reduction‐intention treatment due to haematologic neoplasm died, which explains prognosis being worse in the MPNs/MDSs group. This is an elderly patient cohort, with a trend of worse prognosis in older cases. Contrary to data published in general population studies, 5 in our experience no laboratory finding on admission due to COVID‐19 was related to mortality, except from procalcitonin levels. No differences were found in symptoms at clinical onset between survivors and non‐survivors, but almost all patients who died had developed ARDS during follow‐up. ECOG PS on illness onset or admission appears to be a useful variable associated with worse OS. In the case of our patients, as previously stated, 6 IL‐6 levels usually increase after tocilizumab treatment due to the release of the fraction of IL‐6 bound to its specific receptor. Multivariate analysis suggests that haematologic malignancy status at the time of COVID‐19 is related to mortality, posing a higher risk for patients with active cancer.
Funding information
No financial support was received for this study.
Conflict of interest
All authors declare no conflicts of interest.
Authors contributions
F.M., J.M., M.P. and J.L. conceived of and designed the study. F.M., B.M., A.S., M.C., C.J., B.A. and I.G. contributed to data acquisition, analysis or interpretation. E.R. and C.G. performed IL‐6 quantification. F.M., J.M. and M.P. wrote the manuscript. J.F., P.H. and J.L. were involved in critical revision of the report. All the authors reviewed and approved the final version.
Ethics approval
The study (141/20) was approved by the Clinical Research Ethics Committee, Ramón y Cajal University Hospital in Madrid, Spain.
Supporting information
Table SI. Characteristics and treatment of the global cohort.
Table SII. Univariate analysis of overall survival.
Table SIII. Multivariate analysis of overall survival.
Fig S1. IL‐6 levels pre‐tocilizumab (available in all cases) and 48–72 h after administration (available in 5/8), and survival outcome.
Fig S2. Overall survival of the global cohort.
Acknowledgements
The authors wish to thank Leticia Madrid (lemadrid@ucm.es) for linguistic assistance during the preparation of this manuscript.
References
- 1. Desai A, Sachdeva S, Parekh T, Desai R. COVID‐19 and cancer: lessons from a pooled meta‐analysis. JCO Global Oncol. 2020;6:557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020; 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willan J, King AJ, Hayes S, Collins GP, Peniket A. Care of haematology patients in a COVID‐19 epidemic. Br J Haematol. 2020;189:241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395: 1054–62. 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;29: 105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Characteristics and treatment of the global cohort.
Table SII. Univariate analysis of overall survival.
Table SIII. Multivariate analysis of overall survival.
Fig S1. IL‐6 levels pre‐tocilizumab (available in all cases) and 48–72 h after administration (available in 5/8), and survival outcome.
Fig S2. Overall survival of the global cohort.


