Summary
The goal of assessing tumour response on imaging is to identify patients who are likely to benefit — or not — from anticancer treatment, especially in relation to survival. The World Health Organization was the first to develop assessment criteria. This early score, which assessed tumour burden by standardising lesion size measurements, laid the groundwork for many of the criteria that followed. This was then improved by the Response Evaluation Criteria in Solid Tumours (RECIST) which was quickly adopted by the oncology community. At the same time, many interventional oncology treatments were developed to target specific features of liver tumours that result in significant changes in tumours but have little effect on tumour size. New criteria focusing on the viable part of tumours were therefore designed to provide more appropriate feedback to guide patient management. Targeted therapy has resulted in a breakthrough that challenges conventional response criteria due to the non-linear relationship between response and tumour size, requiring the development of methods that emphasize the appearance of tumours. More recently, research into functional and quantitative imaging has created new opportunities in liver imaging. These results have suggested that certain parameters could serve as early predictors of response or could predict later tumour response at baseline. These approaches have now been extended by machine learning and deep learning. This clinical review focuses on the progress made in the evaluation of liver tumours on imaging, discussing the rationale for this approach, addressing challenges and controversies in the field, and suggesting possible future developments.
Keywords: Liver, Imaging, Tumours, Metastases, RECIST, mRECIST, LI-RADS, EASL
Abbreviations: 18F-FDG, 18F-fluorodeoxyglucose; 90Y, yttrium-90; ADC, apparent diffusion coefficient; APHE, arterial phase hyperenhancement; CEUS, contrast-enhanced ultrasound; CRLM, colorectal liver metastases; DWI, diffusion-weighted imaging; EASL, European Association for the Study of the Liver criteria; GIST, gastrointestinal stromal tumours; HCC, hepatocellular carcinoma; HU, Hounsfield unit; LI-RADS, Liver Imaging Reporting And Data System; (m)RECIST, (modified) Response Evaluation Criteria in Solid Tumours; PET, positron emission tomography; PR, partial response; PD, progressive disease; SD, stable disease; SIRT, selective internal radiotherapy; TR, treatment response; (c)TACE, (conventional) transarterial chemoembolisation; WHO, World Health Organization
Key points.
-
•
The aim of tumour response assessment on imaging is to detect patients that are likely to benefit from anticancer treatment.
-
•
Size-based RECIST criteria, which monitor the progression of entire tumours considered to be representative of tumour burden, are the most extensively used and validated criteria worldwide.
-
•
The mRECIST and EASL criteria were developed to assess tumour response to locoregional treatment targeting hyperenhanced tumours. They introduced the concept of a ‘viable tumour’ and were extended by the LI-RADS treatment response algorithm.
-
•
Criteria focusing on inner tumour modifications rather than changes in size (Choi criteria, Chun criteria) may be more suitable to assess the effect of targeted therapies.
-
•
Quantitative and functional imaging provide insight into microscopic tumour changes that may be used as early predictors of response.
-
•
The goal of 3D criteria is to overcome the limitations of 2D criteria to improve patient classification.
-
•
Deep learning and machine learning create stimulating perspectives for tumour response assessment.
Introduction
The main goal of anticancer treatment is to improve patient survival. Toxicity, adverse events, and changes in quality of life are considered to be ethically acceptable if patients benefit from treatment in the end. However, since not all patients actually do benefit, it is crucial to detect a lack of treatment response both from an oncological, ethical, and socio-economic point of view; although doing so is far from easy. A widely accepted assumption is that tumour burden — i.e. the size of the tumour — is strongly correlated with survival time. From this perspective, monitoring the progression of tumour burden over time can be considered a valid surrogate from the prediction of survival. More simply, tumour response has been assumed to be a strong and valid proxy for increased survival.
The World Health Organization (WHO) criteria for the assessment of tumour response were developed based on this assumption.1 These criteria were rapidly accepted by the oncological community and improvements were made to address their limitations. The Response Evaluation Criteria in Solid Tumours (RECIST) 1.0 — updated as RECIST 1.1. — addresses most of the limitations of the WHO criteria and have become the most widely used and validated set of response criteria in solid tumours worldwide.2,3 They are particularly suited for patients treated with conventional cytotoxic chemotherapy, which mainly includes patients with colorectal metastases and cholangiocarcinoma in the liver.
Conventional chemotherapy regimens play a limited role in other liver tumours, especially hepatocellular carcinoma, and the RECIST criteria cannot reliably determine the oncological benefits of treatments. Indeed, liver tumours are almost exclusively fed by the hepatic artery and are characterized by a rich and a dense network of impaired vessels. This offers a strong rationale for locoregional intra-arterial therapies such as transarterial chemoembolisation (TACE) or radioembolisation. Moreover, numerous molecular treatments target specific biological pathways, such as angiogenesis, tumour metabolism, tumour proliferation, or immune response. All of these therapies, alone or combined, tend to induce necrosis or intratumoural changes that do not necessarily result in tumour shrinkage, leading to an underestimation of tumour response by RECIST.
New generations of imaging-based criteria have been proposed as surrogates for traditional survival-based endpoints that provide a more reliable quantitative assessment of treatment response. These approaches are based on the concept of the ‘viable tumour’, defined as the visualisation of any degree of enhancement after contrast injection. These criteria may be size-based (modified RECIST [mRECIST] and European Association for the Study of the Liver [EASL] criteria4,5) or include the quantification of inner changes in the tumour i (e.g. the Choi criteria6) and have been shown to better identify responders.[7], [8], [9], [10] As a result, certain authors have suggested that some criteria could be used as valid surrogate endpoints for future trials.11
Recently, studies have shown that all the aforementioned criteria fail to effectively take into consideration tumour heterogeneity because they are based on a 2D assessment. Thus, a 3D equivalent of size-based criteria has been proposed that assesses all viable tumour volumes and which seems to be more reliable than 2D criteria.[12], [13], [14]
Quantitative and functional imaging is another stimulating field of research including several techniques that provide information about the physiological properties of tissue on a microscopic level. Diffusion-weighted imaging (DWI), perfusion imaging and metabolic imaging have been shown to successfully detect tumour response earlier than conventional morphological criteria.[15], [16], [17] Studies have even suggested that baseline functional imaging parameters differ between future responders and non-responders,18,19 which could be valuable in adapting treatment, and in planning future management. Nevertheless, functional imaging is still only used for research purposes, due to problems with reproducibility.20,21 This quantitative approach has recently been extended by machine learning and deep learning technologies with promising preliminary results in the assessment of tumour response in the liver.22,23
The aim of this review is to provide a critical overview of the most important imaging-based tumour response criteria in liver tumours. The article focuses on the 3 main hepatic tumours targeted by anticancer treatments, i.e. hepatocellular carcinoma (HCC), hepatic metastases and cholangiocarcinoma. We will follow the historical development from conventional size-based criteria to more recent criteria and discuss their main strengths and limitations.
Imaging modalities
Locoregional and systemic anticancer treatments are mainly evaluated by CT and MRI. Assessment is performed after contrast administration to assess tumour viability, with protocols including a combination of arterial, portal venous and delayed phases, depending on the tumour. Generally, the first evaluation is performed around 4 weeks after the initiation of treatment with follow-up every 3 to 6 months. Although conventional B-mode ultrasound plays an important role in tumour detection, it is marginal when evaluating response. While contrast-enhanced ultrasound (CEUS) is mainly performed for the characterisation of focal liver lesions,24 it has also been shown to be effective in quantifying tumour viability, and studies have suggested that it might be used to monitor patients after ablation,25 or targeted therapies.26 Of note, the performance of ultrasound and CEUS is usually poorer for deep or subdiaphragmatic lesions, especially in obese patients, and in patients with very heterogeneous liver parenchyma. Finally, metabolic imaging with positron emission tomography (PET) is not routinely performed for the assessment of liver tumour response. It may be performed in selected patients (e.g. isolated elevation of tumour markers, doubtful tumour progression, etc).
Whole tumour size-based criteria
The use of objective quantification and tumour size monitoring to define tumour response to cancer treatment is very common in daily practice, for the liver and many other organs. The first criteria were proposed by the WHO in 1979.1 The WHO criteria established a common standard for the identification of tumour response that includes clinical, radiological, biochemical, or surgical-pathological staging. This initiative laid the groundwork for all future imaging-based criteria, which can be summarized in 3 steps:
-
•
Tumours were simplified before assessment. The assessment was based on a 2D measurement of tumours (understood as an approximation of a surface area), calculated by multiplying the maximum diameter by its longest perpendicular diameter.
-
•
The presence of multiple lesions requires the sum of the cross-products of all measured lesions.
-
•
The result is used to classify patients into 4 different categories to evaluate overall tumour response: complete response, partial response, stable disease, and progressive disease (all corresponding to a percentage range of change in the overall tumour burden, Table 1).
Table 1.
Definition of main morphological image-based response criteria.
| WHO | RECIST 1.0 and 1.1 | mRECIST | EASL | Choi | |
|---|---|---|---|---|---|
| Complete response | Disappearance of all lesions | Disappearance of all target lesions (up to 2 measurable liver lesions) RECIST 1.1 added disappearance of pathologic lymph nodes |
Disappearance of any intratumoural arterial enhancement in all target lesion(s) (up to 2 measurable liver lesions) | Disappearance of any intratumoural (arterial and portal) enhancement in all target lesion(s) (up to 2 measurable liver lesions) | Disappearance of all lesions |
| Partial response | ≥50% decrease in sum of cross-product of target lesion(s) | ≥30% decrease in sum of maximum diameter of target lesion(s) | ≥30% decrease in sum of maximum diameter of viable target lesion(s) | ≥50% decrease in total tumour load∗ of all measurable lesion(s) | Decrease in longest diameter ≥10% or in attenuation (HU) ≥15%. No new lesions and no obvious progression of immeasurable disease |
| Stable disease | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD |
| Progressive disease | >25% increase in sum of cross-product of target lesion(s) | >20% increase in sum of diameters. RECIST 1.1 added: must have at least 5 mm absolute increase in sum |
>20% increase in sum of diameters of viable target lesion(s) | ≥25% increase in size of one or more measurable lesion(s) or the appearance of new lesion | Increase in longest diameter ≥10% without meeting tumour attenuation criteria for partial response or the appearance of new lesion |
CR, complete response; HU, Hounsfield unit; PD, progressive disease; PR, partial response; SD, stable disease. Objective response is defined by CR + PR.
Defined as sum of the cross-product of 2 largest diameters or as the sum of surfaces of viable target lesions.
Because of its visionary structure and common language, the WHO criteria rapidly gained wide acceptance among oncologists and were endorsed as the reference criteria to evaluate tumour response in many trials, including in liver tumours.27,28 However, the criteria were found to have certain limitations and needed to be improved. In February 2000 an international collaboration including the European Organization for Research and Treatment of Cancer, the National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group published the RECIST criteria.2 These criteria were further updated and the RECIST 1.1 version was published.3 RECIST is based on the same philosophy as the WHO criteria, with refinements and clarifications such as specifying the minimum target lesion size as well as the maximum number of target lesions per organ and per patient.2,3 The most important change was the use of the sum of the longest diameters of target measurable lesions based on a one-dimensional measurement only. Since then, most clinical trials evaluating liver cancer treatment have used RECIST to define tumour response, including in liver metastases treated with conventional cytotoxic regimens29 (Fig. 1), systemic chemotherapy combined with targeted therapies30,31 or locoregional treatments,32 as well as in primary liver tumours treated with a range of treatments from locoregional33 to systemic.34,35 Recently, large trials including patients with HCC and evaluating radioembolisation,36,37 new targeted therapies,38,39 and even immunotherapy40 have included the RECIST criteria to define tumour response.
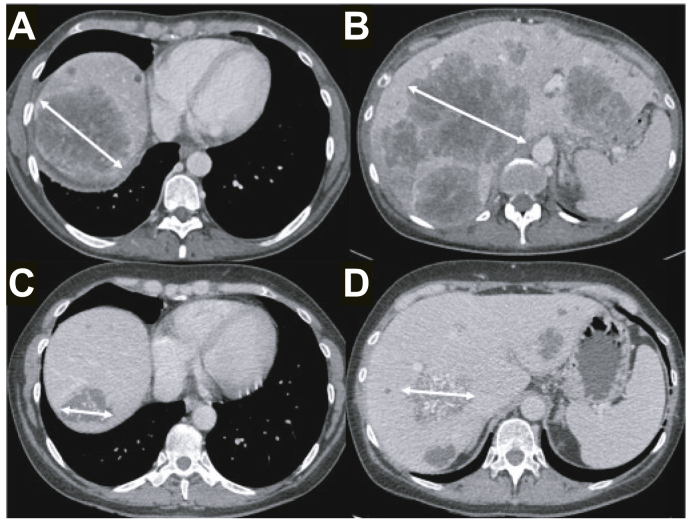
Fig. 1.
55-year-old female patient with rectal cancer.
Baseline contrast-enhanced CT showed bilobar large liver metastases (A and B). Sum of the largest diameters of the 2 target lesions was 242 mm. After 6 cycles of FOLFOX, follow-up contrast-enhanced CT showed a significant decrease in tumour size (C and D), with a sum of the largest diameters of target tumours of 111 mm, corresponding to a 54% decrease. The patient was classified as a partial responder according to RECIST 1.1. Of note, tumours also showed calcifications on follow-up imaging. This is not considered by RECIST but is often associated with a major histological response. RECIST, Response Evaluation Criteria in Solid Tumours.
Overall, RECIST remains the most important and most widely used set of imaging-based tumour response criteria in the liver worldwide. However, despite the progress made with RECIST, it is still associated with several limitations. Certain general limitations concern all organs, for example the assumption that all lesions are spherical and that they will decrease or increase in size uniformly, or the failure to take into consideration the presence of necrosis. Other limitations are liver-specific and due to the specificity of liver tumours.
Most hepatic tumours are characterised by a rich network of arterial vessels that may or may not be impaired. This is often seen on imaging as contrast enhancement in arterial phase images (so-called ‘arterial phase hyperenhancement’ [APHE]). This is mainly true in hepatocellular tumours (especially HCC) and rare forms of secondary tumours (e.g. neuroendocrine tumour metastases). The goal of locoregional treatments (i.e. ablation, intra-arterial therapies), and of several targeted therapies (e.g. anti-antiogenic agents) is to induce tumour necrosis, which is seen as a significant decrease in APHE rather than tumour size or volume. Thus, the RECIST criteria, which only consider lesion shrinkage, largely underestimate tumour necrosis following locoregional treatment,41 and improvement is needed. In other words, these new treatments challenge the definition of tumour response and call for a change in patient assessment.
Viable tumour size-based criteria
Lencioni et al. proposed a modified version of the RECIST criteria (mRECIST) to overcome the drawbacks in the assessment of tumour response in HCC following locoregional treatment.4 EASL also published a new set of criteria (EASL criteria).5 Both of these new criteria introduced the notion of ‘viable tumour’ which corresponds to the portion of tumours showing significant or persistent enhancement after intravenous contrast administration (excluding necrotic [non-enhancing] areas), either on arterial phase (APHE) or venous phase images.
Differences between mRECIST and EASL criteria
Both the mRECIST and EASL criteria are based on the measurement and monitoring of persistently enhanced lesions. However, tumour viability is determined on arterial phase images alone in the mRECIST criteria while EASL criteria can also use portal venous phase images. The mRECIST and RECIST criteria are based on the same philosophy (i.e. identification of target tumours and measurement of the single largest axial diameter of the lesion, classification of patients into 4 categories based on the progression of the sum of the largest diameters over time, etc). The only difference is that mRECIST focuses on the viable (i.e. enhanced) parts of tumours. With EASL criteria the largest axial bidimensional diameters or the enhanced area of the lesion must be measured. This results in different partial response (PR) and progressive disease (PD) thresholds. Details on the assessment of response using mRECIST and EASL are provided in Table 1.
mRECIST and EASL criteria to assess early-intermediate HCC treated with locoregional therapy
Identification of patients with complete or nearly complete tumour necrosis has been shown to be better with mRECIST than with RECIST criteria[42], [43], [44] (Fig. 2). Only a few studies have compared the performance of mRECIST and EASL criteria in the assessment of tumour response after locoregional treatment. A meta-analysis including 7 publications showed no difference between mRECIST and EASL criteria for the assessment of tumour response.45 There was agreement between the 2 criteria in 1,286 out of 1,357 treated patients (95%) — (Kappa 0.93). Moreover, the hazard ratio for overall survival was similar for the 2 criteria (0.39 for mRECIST and 0.38 for EASL criteria).
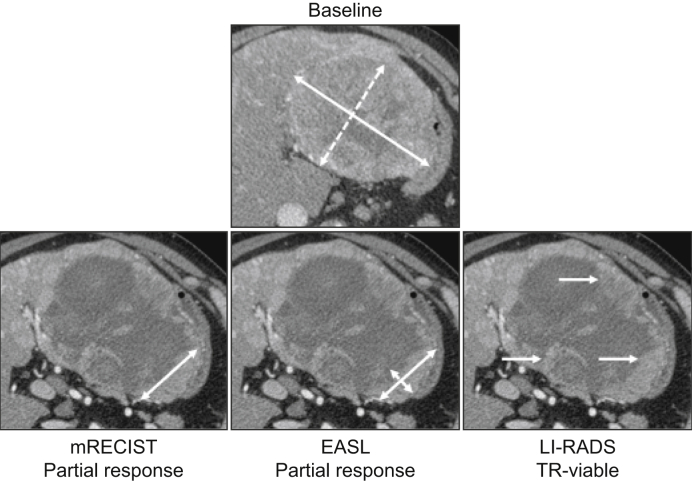
Fig. 2.
62-year-old male patient with a hepatocellular carcinoma developed on HCV-related cirrhosis.
Baseline contrast-enhanced CT (arterial phase) showed a large tumour located in the left liver, with heterogeneous hyperenhancement on arterial phase, consistent with tumour viability. The patient underwent 1 session of chemoembolisation with drug-eluting beads containing idarubicin. One-month follow-up contrast-enhanced CT showed no change in tumour size but significant decrease of viable areas (i.e. showing contrast enhancement). mRECIST showed >30% decrease in the largest diameter of viable areas corresponding to a partial response. EASL criteria showed a >50% decrease in the cross-product of the 2 largest diameters of viable area, also corresponding to a partial response. The persistence of enhancing areas at the periphery of the treated tumour corresponds to TR-viable according to the LI-RADS response algorithm. EASL, European Association for the Study of the Liver criteria; LI-RADS, Liver Imaging Reporting And Data System; (m)RECIST, (modified) Response Evaluation Criteria in Solid Tumours.
Tumour response according to both mRECIST and EASL criteria is known to be associated with overall survival after both thermoablation and TACE.10,41,46,47 They can also be predictive of a good outcome in patients undergoing liver transplantation even in those outside the Milan criteria.48 Most investigators use mRECIST rather than EASL criteria for the assessment of intermediate stage HCC after locoregional treatments, especially in phase II and III trials to compute time-to-progression or time-to-recurrence, because it is simpler and has been found to be reproducible.49,50 In addition, an objective response after TACE according to mRECIST has been suggested as a primary endpoint for future phase II trials even if the level of evidence for this recommendation is weak.51
mRECIST and EASL criteria to assess advanced HCC treated with systemic chemotherapy
Targeted therapies, including — but not limited to — antiangiogenic molecules (e.g. sorafenib, bevacizumab) also induce tumour necrosis, but initially lead to minimal change in tumour size. Therefore, although initially designed to evaluate the response of HCC to locoregional treatment, mRECIST criteria have been shown to be better than RECIST for advanced HCCs treated with systemic therapies.52,53 Convincing evidence, mostly in sorafenib-treated patients from retrospective and randomized controlled trials, has shown that the mRECIST response should be considered an independent prognostic factor.11,[54], [55], [56] Accordingly, drugs that result in high response rates can be expected to prolong overall survival in many patients.
Limitations of mRECIST and EASL criteria
Conventional chemoembolisation
Conventional chemoembolisation (cTACE) involves the intrahepatic transarterial delivery of an emulsion of a concentrated chemotherapeutic agent and ethiodized oil (Lipiodol; Laboratoire Guerbet; Villepinte; France). It is one of the most frequently used treatments for HCC. Both mRECIST and EASL criteria assume that tumour areas containing lipiodol are necrotic, i.e. show no contrast enhancement. However, due to its spontaneous hyperattenuation on CT, lipiodol deposits can partially mask persistently hyperenhanced portions of tumours, which could explain a tendency to overestimate tumour response after cTACE with mRECIST.[42], [43], [44] Certain authors suggest using MRI, which is insensitive to lipiodol, to overcome this limitation.57 At present, the evidence supporting the superiority of MRI over CT is limited to small retrospective series.58,59 It is interesting to note that several studies have reported a strong correlation between the amount of lipiodol deposition, the extent of tumour necrosis, and the degree of tumour devascularisation (Fig. 3).[60], [61], [62], [63] We have shown that the combination of a complete response according to mRECIST/EASL criteria and a homogenous lipiodol deposition pattern helps identify tumours with major necrosis.43 Kinugasa et al. also showed, and we recently confirmed, that heterogeneous lipiodol deposition is associated with a high risk of recurrence.64,65
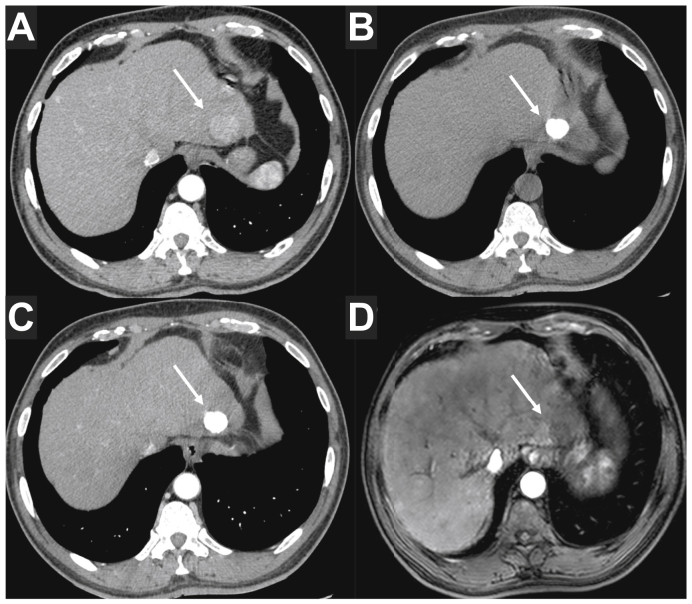
Fig. 3.
59-year-old male patient with hepatocellular carcinoma developed on HCV-related cirrhosis.
Baseline contrast-enhanced CT (A) showed a solitary 25 mm HCC located in segment 2 (arrow). The patient underwent 1 session of selective conventional TACE with idarubicin as a bridge to liver transplantation. Follow-up CT performed 4 weeks after the TACE session showed a dense and homogeneous lipiodol deposition in the tumour on precontrast images (arrow in B), without persistent APHE (arrow in C). The lesion was classified as a complete response according to mRECIST and EASL criteria, and LR non-viable according to the LI-RADS treatment response algorithm. Although the lipiodol deposition could be considered to prevent an accurate tumour response assessment, this pattern of lipiodol deposition has been shown to be consistent with a major pathological response. MRI is insensitive to the presence of lipiodol and a follow-up contrast-enhanced MRI was performed and confirmed the absence of APHE (arrow in D). The patient underwent liver transplantation, and pathological analysis showed close to 100% necrosis in the treated tumour. APHE, arterial phase hyperenhancement; EASL, European Association for the Study of the Liver criteria; HCC, hepatocellular carcinoma; LI-RADS, Liver Imaging Reporting And Data System; (m)RECIST, (modified) Response Evaluation Criteria in Solid Tumours; TACE, transarterial chemoembolisation.
Non-measurable lesions: Infiltrative/non-hyperenhanced HCC
HCC may present as an ill-defined lesion with infiltrative margins and develop with a predominantly intravascular growth pattern. These forms of the disease are often non-hyperenhanced on arterial phase images. In these cases, mRECIST and EASL criteria can still be applied to assess tumour response according to RECIST criteria, but the tumour should not be considered as the target lesion.
Delayed response after radioembolisation
Selective internal radiotherapy (SIRT) with yttrium-90 (90Y), also known as radioembolisation, is the other locoregional therapy used in HCC. This treatment results in tumour necrosis so mRECIST or EASL criteria should be applied rather than RECIST criteria to evaluate treatment response, identify responders and predict patient outcomes.66 However, post-therapeutic fibrosis and inflammation, as well as delayed shrinkage of viable parts of the tumour, are challenges with these criteria.67
Viable tumour appearance-based criteria
Treatment efficacy can also be evaluated based on the appearance of viable parts of the tumour rather than its size on imaging. This approach has been introduced to improve the assessment of response to locoregional treatments as well as to adapt to the differences in treatment response following targeted therapies.
The use of targeted cancer therapies is relatively recent in clinical practice. These approaches block the progression of cancer by interfering with specific pathways involved in tumour growth and have significantly changed cancer treatment in the past 20 years. Their mechanisms of action are different from those of traditional cytotoxic chemotherapy. Certain drugs induce apoptosis, while others focus on proteins that are involved in cell signalling pathways, which form a communication system that governs cellular functions and activities. Because of these pharmacodynamic differences, tumours treated with targeted therapies do not necessarily demonstrate the same imaging findings as those treated with conventional cytotoxic therapies. The first tumours treated with targeted therapies were gastrointestinal stromal tumours (GIST), HCC, and melanoma. Conventional response criteria were disappointing in these cases with a risk of misclassification, and bias due to the unchanged size of the tumour, even though there was a marked change in the appearance of the tumour.
Choi criteria
The Choi criteria were developed when imatinib, a tyrosine kinase receptor inhibitor, changed the management of patients with advanced metastatic GIST. It is interesting to note that there was no linear correlation between treatment efficacy and the size of the tumour in these cases. Indeed, certain patients who responded to treatment demonstrated a paradoxical increase in tumour size due to various histological changes such as haemorrhage, necrosis or myxoid degeneration. Based on these findings, Choi et al. proposed a new set of response criteria based on the modification of CT tumour attenuation and size, that were initially developed for GIST tumours.6 Response could be detected shortly after treatment initiation with these criteria. Based on the validation in GIST tumours, the Choi criteria have also been studied for other hyperenhanced tumours, including metastatic renal cell carcinoma,68 non-GIST sarcomas, neuroendocrine tumours (Fig. 4), and finally HCC.7 Indeed, treatment response defined by the Choi criteria has been associated with longer survival in patients with advanced HCC treated with sorafenib,7,52 as well as in patients with intrahepatic cholangiocarcinoma treated with SIRT.69
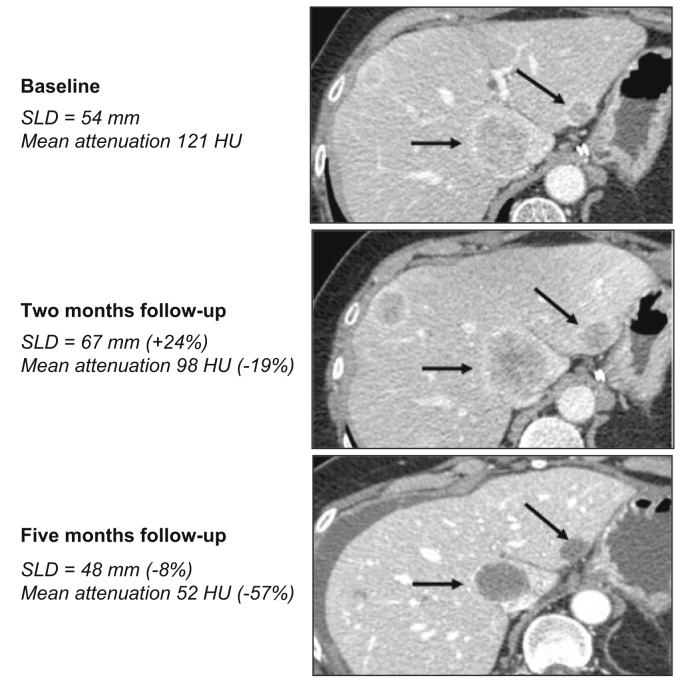
Fig. 4.
47-year-old female patient with neuroendocrine liver metastases (pancreatic origin).
Baseline contrast CT (portal venous phase) shows bilobar heterogeneous liver secondary tumours. Target lesions are indicated by black arrows. The patient received sunitinib, a multikinase inhibitor. Two-month follow-up CT (portal venous phase) showed +24% increase in the sum of largest diameters of target lesions, corresponding to progressive disease as per the RECIST 1.1 criteria. Mean target tumour attenuation was a mean −19% lower, corresponding to an objective response according to the Choi criteria. Five-month follow-up CT confirmed the objective response according to the Choi criteria (mean −57% in tumour attenuation) while tumours were considered as stable disease according to RECIST 1.1 (sum of largest diameters of target lesions −8%). RECIST, Response Evaluation Criteria in Solid Tumours.
However, the Choi criteria have certain limitations for the evaluation of HCC response. Lipiodol deposition results in an unreliable measurement of tumour attenuation in patients treated by cTACE,43 while post-treatment changes, especially fibrosis, interfere with a reliable assessment in HCC treated with radioembolisation.70 Finally, the inter-reader agreement on Choi's response criteria for HCC was found to be moderate.7
LI-RADS CT/MRI treatment response algorithm
The Liver Imaging Reporting And Data System (LI-RADS) is a comprehensive system to standardize the terminology, technique, interpretation, reporting, and data collection of liver imaging.71 The LI-RADS system was designed to improve communication, patient care, education, and research. It is consistent with and fully integrated into the American Association for the Study of Liver Diseases (AASLD) clinical practice guidance.72 The LI-RADS includes a treatment response algorithm that can be applied to patients with liver malignancies treated by ablation, intra-arterial therapies or external beam radiation therapy. The algorithm is based on the visual assessment of tumour viability defined as nodular, mass-like, or thick, irregular tissue in or along the treated lesion showing APHE or washout appearance, or an enhancement similar to that observed before treatment.73 The algorithm differentiates viable tumours (named LR TR-viable), from non-viable ones (LR TR-non-viable) (Fig. 2). It also includes the category of “equivocal enhancement” when the tumour shows atypical enhancement that does not meet criteria for probable or definite viability. Viable portions may be measured, but measurement is not required to assess tumour response. Shropshire et al. have reported high predictive values with moderate inter-reader association for the histopathologic viability of HCC treated with bland arterial embolisation.74 Seo et al. further suggested that the diagnostic performance of the LI-RADS treatment response algorithm was better on CT.75 When LI-RADS criteria are applied, CT and MRI are comparable for the diagnosis of HCC tumour viability after locoregional treatment.
MD Anderson's morphological criteria
MD Anderson's morphological criteria, also known as Chun criteria, were initially created to evaluate the response to bevacizumab (a monoclonal antibody targeting vascular endothelial growth factor) in patients with metastatic colorectal cancer.76 These CT-based criteria are based on 3 main characteristics: lesion attenuation, interface lesion-liver and the presence of rim enhancement. The combination of these criteria enables tumours to be stratified into 3 response categories (“optimal”, “suboptimal” and “no response”), and has been shown to correlate with pathological response and survival77 (Fig. 5). The same team that initially proposed the Chun criteria also explored their use in patients who received preoperative systemic chemotherapy with or without bevacizumab for liver colorectal metastases, and proposed their use as a surrogate therapeutic endpoint in this indication.78 Indeed, one advantage of these criteria is the inclusion of the tumour-liver interface whose thickness has been shown to be inversely correlatedwith recurrence-free survival.79
Fig. 5.
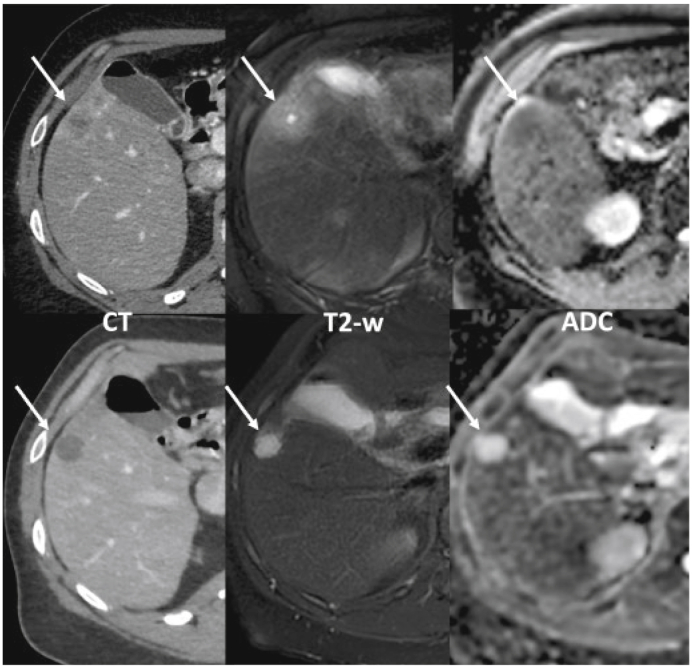
62-year-old male patient with left colon cancer and liver metastasis in segment 5 (arrows).
The upper row shows baseline contrast-enhanced CT (left) and MRI (centre T2-weighted and right ADC map). The lower row shows follow-up exams after 6 cycles of FOLFOX. On baseline, the lesion appears ill-defined and heterogeneous, with peripheral contrast uptake. After chemotherapy, the lesion is homogeneously hypoattenuating, with sharp border, and no peripheral contrast enhancement, thus corresponding to an ‘optimal response’ according to the MD Anderson criteria. It corresponds to a shift from baseline mild and heterogeneous signal hyperintensity to a homogeneous high signal intensity on T2-w images. ADC maps show a significant increase in the ADC values of the tumour under chemotherapy, also consistent with tumour response. ADC, apparent diffusion coefficient.
All the viable tumour appearance-based criteria mentioned above have a similar limitation: it takes weeks and sometimes months to confidently confirm the response. Earlier assessment of tumour response could improve patient management and perhaps offer more cost-effective treatment strategies.
Functional imaging
Functional imaging refers to various techniques that provide information about the physiological properties of tissue at a microscopic level. The main techniques in the liver include:
-
•
DWI, which is sensitive to the Brownian motion of water molecules and has been confirmed as a valid marker of tissue cellularity and microarchitecture.15 This technique provides information on the degree of diffusion restriction in tissue through both a qualitative visual assessment and quantitative measurements of the apparent diffusion coefficient (ADC).
-
•
Perfusion imaging, which uses CEUS, CT or MRI to provide information about tissue microcirculation, i.e. micro-movement of water and solutes.
-
•
Metabolic imaging using PET/CT or PET/MRI with dedicated targeted radiotracers to assess specific metabolic pathways.
-
•
Imaging of hepatocellular function using hepatospecific MR contrast agents.
The first 3 techniques are used to assess tumour response and will be discussed here. The last technique is mainly used for tumour detection and characterisation, and for the quantification of liver function reserve and will not be described in this review. The use of functional imaging techniques to assess tumour response is based on 3 hypotheses:
-
•
First, treatment-induced tissue modification (e.g. necrosis, apoptosis, devascularisation, etc) results in changes in imaging parameters that can be objectively quantified.
-
•
Second, there is a positive correlation between the extent of tissue change and changes in imaging features. Thus, tumours with a better response are expected to have more significant changes in imaging features.
-
•
Baseline imaging features are different in tumours that will have an objective response and those that will not.
This explains why the literature can be separated into 3 main categories. The first and largest body of literature includes studies showing that functional imaging confirms assessments using morphological criteria (e.g. RECIST or mRECIST). The added value of functional imaging is limited in these cases. The second group includes research showing that functional imaging can identify responders significantly earlier than morphological criteria, which may have clinical value to determine treatment strategies. Finally, there is a group of studies showing that baseline functional imaging parameters differ in future responders and non-responders. Although this latter group is the smallest, it is highly interesting and could represent a significant change in patient management.
Diffusion-weighted Imaging
ADC has mainly been studied in primary tumours for tumour detection and characterisation and is now routinely used for this purpose.15 Nevertheless, a quantitative approach has been studied to assess tumour response and several pre-clinical and clinical studies have shown that quantification of ADC could help estimate the degree of tumour necrosis in HCC treated with locoregional therapy, since necrotic tissue shows higher ADC values than viable tumours.16,[80], [81], [82], [83] It is important to note that these modifications can be observed early, within a week after treatment.18,19 Researchers have also investigated the role of baseline pre-treatment ADC values in predicting future tumour response. These limited and retrospective series have shown that ADC values in tumours before TACE or radioembolisation can be used to predict tumour response and patient survival.[84], [85], [86] Similar results have been reported with mass-forming cholangiocarcinoma treated with intra-arterial therapy17 and with liver metastases (Fig. 5).
Perfusion imaging
Other studies have shown that perfusion imaging techniques are of limited value after percutaneous microwave or radiofrequency ablation because morphological criteria are sufficiently reliable for assessing tumour response and recurrence.41,87 One study using perfusion CT suggested that quantification of blood volume could be useful in detecting local progression in close contact with ablation areas,88 but these results have not been confirmed. Perfusion imaging has more frequently been studied to assess the efficacy of intra-arterial therapy. Once again, pre-clinical studies showed that early changes in perfusion parameters can be observed within 1 week after TACE in treated tumours.[89], [90], [91] Similar results have been reported in patients.20,92 Pre-treatment perfusion parameters before TACE have also been shown to help predict progression-free survival, regardless of the number of lesions or tumour size. In patients treated with targeted therapies, perfusion parameters decrease early and significantly in responders but not in non-responders. Higher baseline perfusion values are observed in patients whose disease is controlled by treatment.93,94 French multicentre studies have also shown that quantitative CEUS examinations could help predict tumour progression using a standardised approach.26,95
Similar results have been published in liver metastases, especially of a colorectal origin. Perfusion imaging has been studied to assess the response of colorectal liver metastases (CRLM) to treatment with combined cytotoxic and targeted therapies. Baseline vascular permeability was shown to be significantly higher in responders and it was also shown to significantly decrease after 6 weeks of treatment.96 Moreover, patients with >40% decrease in the transfer constant using perfusion MRI had better progression-free survival.97 Significant differences in arterial perfusion have been observed between responders and non-responders to radioembolisation on pre-treatment perfusion CT,21 with higher perfusion associated with improved 1-year survival.
Metabolic imaging
The use of 18F-fluorodeoxyglucose (18F-FDG) PET is not systematically recommended in CRLM to monitor chemotherapy. Chemotherapeutic MRI is preferred in patients with resectable metastases, especially in the neoadjuvant setting. Limited data suggest that 18F-FDG PET/CT may be useful after radioembolisation in non-surgical patients. An early metabolic response defined as a >50% reduction in the liver-to-tumour ratio on 18F-FDG PET may be correlated to post-treatment survival and could help determine treatment options and follow-up management.98 Furthermore, 18F-FDG PET/CT-derived factors such as functional tumour volume and total lesion glycolysis have been shown to be significant predictors of patient survival following radioembolisation in small series.
Despite these promising results, functional imaging techniques are rarely used in daily practice to assess tumour response. This is mainly due to certain limitations in these techniques such as suboptimal reproducibility, lack of standardisation, influence of mathematical models, and post-processing. This results in high variability and difficulty in replicating and comparing results between different devices and teams.
Future perspectives
iRECIST
Recently targeted therapy has begun focusing on the development of immunomodulating drugs with novel mechanisms of action based on the activation of immune cells. In contrast to previous developments in tumour evaluation that have mainly involved refinements in the definition of tumour response, these molecules are associated with possible unusual patterns of progression, that was called “pseudo-progression” in early trials of immune-based therapeutics in melanoma. This includes an increase in the size of lesions, or the visualisation of new lesions, followed by a possible durable response. Several sets of criteria have been proposed to cope with these observations. The most recent are the iRECIST criteria that have been developed by the RECIST Working Group, pharmaceutical companies, regulatory authorities and academia to ensure consistent design and data collection. iRECIST is based on RECIST 1.1 and has been extensively used in a number of clinical trials on immunotherapeutic drugs.99 Although the first few studies using iRECIST to evaluate treatment response in liver cancer are now being published,100 their reliability has not yet been validated. Consequently, the best criteria to assess patients receiving immunotherapy is still unknown. This is important as immunotherapy is progressively being included in treatment algorithms for liver tumours, especially HCC. To make things more complicated, properly monitoring patients treated with combinations of immunomodulating and antiangiogenic agents (e.g. atezolizumab and bevacizumab in advanced HCC), will require imaging criteria designed to consider both the change in tumour response (induced by anti-angiogenic molecules) and in tumour progression (possibly associated with immunomodulating agents), resulting in deeply modified versions of known criteria. The resulting “miRECIST” criteria remain to be defined.
Volumetric analysis
All previously mentioned 2D measurement methods (RECIST, mRECIST, Choi, etc) have inherent limitations. For example, the slices used to assess response are considered to be surrogates of overall tumour volume, although they may not reliably reflect this.101 Indeed, not all tumours are spherical and linear measurements can be difficult due to irregular shapes or complex morphologies.102 Moreover, liver tumours tend to demonstrate skewness and inhomogeneous enhancement patterns, especially after treatment, as some tumours do not expand or shrink uniformly.14 Tumours often grow asymmetrically with different areas changing at different rates. In these cases, the size (volume) of a tumour may increase while the longest diameter remains unchanged. Furthermore, 2D methods require the radiologist to choose one representative section on cross-sectional imaging, resulting in a high inter- and intra- observer variability.103,104
The development of new automated or semi-automated tumour segmentation tools, either in-house or in the form of commercial software, to determine the actual extent and distribution of tumour tissue, has paved the way for 3D tumour imaging quantification. Most of these segmentation tools are based on a manually drawn region of interest around the tumour followed by a computerised volume calculation.16 Several studies have demonstrated the reliability of semi-automatic CT or MR-based 3D methods to predict response and survival in a broad morphological variety of tumours, regardless of tumour shape or enhancement distribution.12,13,105 This has led to the development of volumetric RECIST and quantitative EASL criteria.106 One of the main advantages of these volume-based methods is that they detect tumour changes earlier and with greater sensitivity than simpler 2D morphological methods, allowing early stratification of patient response12 (Fig. 6). These 3D techniques have also been studied to predict the response to treatment.13,107
Fig. 6.
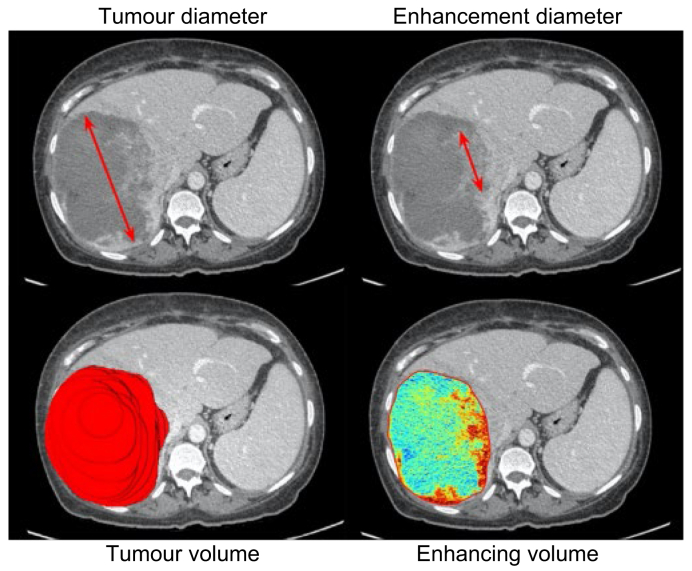
Comparison of 2D and 3D assessment techniques.
57-year-old female patient with a large hepatocellular carcinoma developed on HBV-related non-cirrhotic liver. The patient underwent 2 sessions of chemoembolisation with drug-eluting beads containing doxorubicin. One-month follow-up contrast-enhanced CT (portal phase). (A) Unidimensional measurement of the largest lesion diameter. (B) Unidimensional measurement of the largest enhancing diameter (i.e., viable tissue). (C) Segmentation-based tumour volume. (D) Quantification of enhancing lesion volume, with read areas indicating area of maximum enhancement or viable tissue.
However, despite the conceptual advantage, the accuracy of the 3D response methods is also influenced by technical parameters. In particular, they are affected by small or irregularly shaped lesions and require high-quality image-contrast examinations. Standardisation is needed, as there are many existing 3D segmentation tools, and their use in daily practice is still limited. Finally, these techniques have not received regulatory approval, from the U.S. Food and Drug Administration, for instance, which limits their use as primary endpoints in phase III trials.
Machine learning (radiomics) and deep learning
Machine learning and deep learning have captured the attention and imagination of the medical community. Machine learning refers to a subcategory of artificial intelligence research that generates abstracted models with computers via data and observations.108 Deep learning refers to a subfield of machine learning which relies on multiple processing layers to learn generalisable representations of data with higher levels of abstraction.109,110 The increased interest in these techniques combined with the massive accumulation of multiparametric imaging, pathology, laboratory and clinical data, has resulted in a groundswell of research.
Among machine learning techniques, radiomics, which was presented in 2012 by Lambin et al.,111 has raised expectations. Radiomics manipulates images on a voxel level, with the goal of going beyond size or human-eye based semantic descriptors of tumours, to enable the non-invasive extraction of quantitative radiological data from medical images and to explore their correlation with clinical outcomes or pathological characteristics.22 This approach would quantify the morphological aspects of the tumour, and evaluate its heterogeneity through mathematically defined features, with a final objective of generating imaging phenotypes.112 The typical radiomics analysis pipeline consists of 3 main steps: i) tumour segmentation, ii) computation of radiomic features within the segmented region, and iii) feature selection, model building and classification.
Radiomic methods are not only designed to predict early overall survival113 or to identify predictive pathological characteristics such as microvascular invasion,23 they may also predict liver tumour response to treatment.114,115 Indeed, there is early evidence that pre-treatment CT-derived signatures can predict survival in patients with resected HCC116 or advanced HCC treated with sorafenib.117
Nevertheless, radiomics is a recent method that requires further development to overcome limitations inherent to complex, computer-dependent models, in particular, the lack of standardisation of image acquisition such as reconstruction kernel or section thickness, which can obscure important underlying biological texture features.118 To address this issue, post-processing techniques are being studied to compensate for variations in radiomic feature values caused by different CT protocols.119
To date, evidence of deep learning in liver imaging120,121 and radiology is very limited and the performance has not been validated. There is much to learn about and from deep learning, and its potential applications. Recent network architectures, mainly from non-medical competitions (e.g. Common Objects in Context challenge), are particularly interesting as they combine the ability of localisation, segmentation and classification tasks in a fast-computational time. Due to the absence of visible rules used by neural networks, strong guidelines are needed to direct research in deep learning so that it can fulfil what could be a significant role in the field of tumour assessment.
Conclusion
The RECIST 1.1 criteria remain the reference for both clinicians and researchers. They routinely guide standard patient care and have been used to define reference endpoints in trials, such as treatment response/progression rate or time-to-progression, whatever the stage of development of new anticancer therapeutics. Nevertheless, the regular development of new treatments has resulted in previously undescribed modifications of the tumour on imaging, and requires new, suitable, validated image-based evaluation criteria of tumour response. Criteria focusing on tumour viability, especially the mRECIST criteria, have gradually become recognised by clinicians and regulatory authorities and may become the most valid alternative to RECIST in the years to come. On the other side of the spectrum, the recent introduction of immunomodulating drugs has challenged the definition of progression. New sets of criteria, such as iRECIST, have been introduced and will require validation.
From a very different perspective, innovations involving the combination of functional or quantitative imaging, 3D assessment and deep learning (“deep volumic functional imaging” or “deep volumic radiomics”) could lead to disruptive approaches in this field. No longer dependent on human image processing and based on the networks' ability to process highly complex data representations, these innovations could provide further progress in the field of personalised oncology. Whether current efforts to build huge databanks will overcome the challenges of reproducibility and interpretability, remains a key question.
Financial support
The authors received no financial support to produce this manuscript.
Authors' contributions
Conceptualisation: MR. Validation: MR. Writing-original draft: All authors. Writing-review & editing: All authors.
Conflict of interest
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100100.
Supplementary data
References
- 1.Organization W.H. World Health Organization; sold by WHO Publications Centre USA; Geneva and Albany, NY: 1979. WHO Handbook for Reporting Results of Cancer Treatment. [Google Scholar]
- 2.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M., Llovet J.M., Beaugrand M., Lencioni R., Burroughs A.K. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 6.Choi H., Charnsangavej C., Faria S.C., Macapinlac H.A., Burgess M.A., Patel S.R. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 7.Ronot M., Bouattour M., Wassermann J., Bruno O., Dreyer C., Larroque B. Alternative response criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394–402. doi: 10.1634/theoncologist.2013-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prajapati H.J., Spivey J.R., Hanish S.I., El-Rayes B.F., Kauh J.S., Chen Z. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE) Ann Oncol. 2013;24:965–973. doi: 10.1093/annonc/mds605. [DOI] [PubMed] [Google Scholar]
- 9.Edeline J., Boucher E., Rolland Y., Vauléon E., Pracht M., Perrin C. Comparison of tumor response by response evaluation criteria in solid tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 10.Gillmore R., Stuart S., Kirkwood A., Hameeduddin A., Woodward N., Burroughs A.K. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–1316. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R., Montal R., Torres F., Park J.W., Decaens T., Raoul J.L. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017;66:1166–1172. doi: 10.1016/j.jhep.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Tacher V., Lin M., Duran R., Yarmohammadi H., Lee H., Chapiro J. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. 2016;278:275–284. doi: 10.1148/radiol.2015142951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapiro J., Duran R., Lin M., Schernthaner R., Lesage D., Wang Z. Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol. 2015;25:1993–2003. doi: 10.1007/s00330-015-3595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapiro J., Lin M., Duran R., Schernthaner R.E., Geschwind J.F. Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert Rev Anticancer Ther. 2015;15:199–205. doi: 10.1586/14737140.2015.978861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taouli B., Koh D.M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 16.Bonekamp S., Jolepalem P., Lazo M., Gulsun M.A., Kiraly A.P., Kamel I.R. Hepatocellular carcinoma: response to TACE assessed with semiautomated volumetric and functional analysis of diffusion-weighted and contrast-enhanced MR imaging data. Radiology. 2011;260:752–761. doi: 10.1148/radiol.11102330. [DOI] [PubMed] [Google Scholar]
- 17.Halappa V.G., Bonekamp S., Corona-Villalobos C.P., Li Z., Mensa M., Reyes D. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264:285–294. doi: 10.1148/radiol.12112142. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.Y., Li C.W., Kuo Y.T., Jaw T.S., Wu D.K., Jao J.C. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants - initial experience. Radiology. 2006;239:448–456. doi: 10.1148/radiol.2392042202. [DOI] [PubMed] [Google Scholar]
- 19.Chung J.C., Naik N.K., Lewandowski R.J., Deng J., Mulcahy M.F., Kulik L.M. Diffusion-weighted magnetic resonance imaging to predict response of hepatocellular carcinoma to chemoembolization. World J Gastroenterol. 2010;16:3161–3167. doi: 10.3748/wjg.v16.i25.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson A.C., Wang D., Atassi B., Sato K.T., Ryu R.K., Lewandowski R.J. Transcatheter intraarterial perfusion: MR monitoring of chemoembolization for hepatocellular carcinoma - feasibility of initial clinical translation. Radiology. 2008;246:964–971. doi: 10.1148/radiol.2463070725. [DOI] [PubMed] [Google Scholar]
- 21.Morsbach F., Pfammatter T., Reiner C.S., Fischer M.A., Sah B.R., Winklhofer S. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol. 2013;48:787–794. doi: 10.1097/RLI.0b013e31829810f7. [DOI] [PubMed] [Google Scholar]
- 22.Aerts H.J. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol. 2016;2:1636–1642. doi: 10.1001/jamaoncol.2016.2631. [DOI] [PubMed] [Google Scholar]
- 23.Xu X., Zhang H.L., Liu Q.P., Sun S.W., Zhang J., Zhu F.P. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133–1144. doi: 10.1016/j.jhep.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Trillaud H., Bruel J.-M., Valette P.-J., Vilgrain V., Schmutz G., Oyen R. Characterization of focal liver lesions with SonoVue®-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748. doi: 10.3748/wjg.15.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lekht I., Gulati M., Nayyar M., Katz M.D., Ter-Oganesyan R., Marx M. Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom Radiol. 2016;41:1511–1521. doi: 10.1007/s00261-016-0700-4. [DOI] [PubMed] [Google Scholar]
- 26.Lassau N., Bonastre J., Kind M., Vilgrain V., Lacroix J., Cuinet M. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794–800. doi: 10.1097/RLI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominguez S., Denys A., Madeira I., Hammel P., Vilgrain V., Menu Y. Hepatic arterial chemoembolization with streptozotocin in patients with metastatic digestive endocrine tumours. Eur J Gastroenterol Hepatol. 2000;12:151–157. doi: 10.1097/00042737-200012020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gerard A., Buyse M., Pector J.C., Bleiberg H., Arnaud J.P., Willems G. Hepatic artery ligation with and without portal infusion of 5-FU. A randomized study in patients with unresectable liver metastases from colorectal carcinoma. The E.O.R.T.C. Gastrointestinal Cancer Cooperative Group (G.I. Group) Eur J Surg Oncol. 1991;17:289–294. [PubMed] [Google Scholar]
- 29.Nordlinger B., Sorbye H., Glimelius B., Poston G.J., Schlag P.M., Rougier P. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primrose J., Falk S., Finch-Jones M., Valle J., O'Reilly D., Siriwardena A. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 31.Stintzing S., Fischer von Weikersthal L., Decker T., Vehling-Kaiser U., Jager E., Heintges T. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23:1693–1699. doi: 10.1093/annonc/mdr571. [DOI] [PubMed] [Google Scholar]
- 32.Wasan H.S., Gibbs P., Sharma N.K., Taieb J., Heinemann V., Ricke J. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–1171. doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llovet J.M., Real M.I., Montana X., Planas R., Coll S., Aponte J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 34.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 35.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 36.Vilgrain V., Pereira H., Assenat E., Guiu B., Ilonca A.D., Pageaux G.P. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 37.Chow P.K.H., Gandhi M., Tan S.B., Khin M.W., Khasbazar A., Ong J. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 38.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 39.Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29—Identifier NCT02702401. Study of Pembrolizumab (MK-3475) vs. Best Supportive Care in Participants With Previously Systemically Treated Advanced Hepatocellular Carcinoma (MK-3475-240/KEYNOTE-240); 2016 March 8 [cited 2019 Dec 3]; [about 4 screens]. Available at: https://clinicaltrials.gov/ct2/show/NCT02702401. [Accessed 10 March 2020].
- 41.Forner A., Ayuso C., Varela M., Rimola J., Hessheimer A.J., de Lope C.R. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 42.Bargellini I., Bozzi E., Campani D., Carrai P., De Simone P., Pollina L. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol. 2013;82:e212–e218. doi: 10.1016/j.ejrad.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Dioguardi Burgio M., Ronot M., Bruno O., Francoz C., Paradis V., Castera L. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016;22:1491–1500. doi: 10.1002/lt.24615. [DOI] [PubMed] [Google Scholar]
- 44.Riaz A., Memon K., Miller F.H., Nikolaidis P., Kulik L.M., Lewandowski R.J. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol. 2011;54:695–704. doi: 10.1016/j.jhep.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vincenzi B., Di Maio M., Silletta M., D'Onofrio L., Spoto C., Piccirillo M.C. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS One. 2015;10:e0133488. doi: 10.1371/journal.pone.0133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Memon K., Kulik L., Lewandowski R.J., Wang E., Riaz A., Ryu R.K. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141:526–535. doi: 10.1053/j.gastro.2011.04.054. 535.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sala M., Llovet J.M., Vilana R., Bianchi L., Solé M., Ayuso C. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 48.Bargellini I., Vignali C., Cioni R., Petruzzi P., Cicorelli A., Campani D. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan criteria--selection parameter for liver transplantation. Radiology. 2010;255:289–300. doi: 10.1148/radiol.09090927. [DOI] [PubMed] [Google Scholar]
- 49.Sato Y., Watanabe H., Sone M., Onaya H., Sakamoto N., Osuga K. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16–22. doi: 10.3109/03009734.2012.729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pallan P., Wholey M., Palacios R., Lutz J., Mendez Castillo A., Mehta A. Transarterial chemoembolization with 40-micron drug-eluting beads: a multicenter study, a San Antonio experience. J Vasc Interv Radiol. 2018;29:S22. [Google Scholar]
- 51.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Gavanier M., Ayav A., Sellal C., Orry X., Claudon M., Bronowicki J.P. CT imaging findings in patients with advanced hepatocellular carcinoma treated with sorafenib: alternative response criteria (Choi, European Association for the Study of the Liver, and modified Response Evaluation Criteria in Solid Tumor (mRECIST)) versus RECIST 1.1. Eur J Radiol. 2016;85:103–112. doi: 10.1016/j.ejrad.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 53.Takada J., Hidaka H., Nakazawa T., Kondo M., Numata K., Tanaka K. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609. doi: 10.1186/s13104-015-1565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer T., Palmer D.H., Cheng A.L., Hocke J., Loembe A.B., Yen C.J. mRECIST to predict survival in advanced hepatocellular carcinoma: analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017;37:1047–1055. doi: 10.1111/liv.13359. [DOI] [PubMed] [Google Scholar]
- 55.Kudo M., Ueshima K., Yokosuka O., Ogasawara S., Obi S., Izumi N. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 56.Kudo M. Objective response by mRECIST is an independent prognostic factor of overall survival in systemic therapy for hepatocellular carcinoma. Liver cancer. 2019;8:73–77. doi: 10.1159/000497460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kloeckner R., Otto G., Biesterfeld S., Oberholzer K., Dueber C., Pitton M.B. MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:532–540. doi: 10.1007/s00270-009-9728-y. [DOI] [PubMed] [Google Scholar]
- 58.Hunt S.J., Yu W., Weintraub J., Prince M.R., Kothary N. Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol. 2009;20:30–38. doi: 10.1016/j.jvir.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 59.Kim S., Mannelli L., Hajdu C.H., Babb J.S., Clark T.W., Hecht E.M. Hepatocellular carcinoma: assessment of response to transarterial chemoembolization with image subtraction. J Magn Reson Imaging. 2010;31:348–355. doi: 10.1002/jmri.22038. [DOI] [PubMed] [Google Scholar]
- 60.Kwan S.W., Fidelman N., Ma E., Kerlan R.K., Jr., Yao F.Y. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727–736. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shim J.H., Han S., Shin Y.M., Yu E., Park W., Kim K.M. Optimal measurement modality and method for evaluation of responses to transarterial chemoembolization of hepatocellular carcinoma based on enhancement criteria. J Vasc Interv Radiol. 2013;24:316–325. doi: 10.1016/j.jvir.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 62.Takayasu K., Arii S., Matsuo N., Yoshikawa M., Ryu M., Takasaki K. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704. doi: 10.2214/ajr.175.3.1750699. [DOI] [PubMed] [Google Scholar]
- 63.Vogl T.J., Trapp M., Schroeder H., Mack M., Schuster A., Schmitt J. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 64.Kinugasa H., Nouso K., Takeuchi Y., Yasunaka T., Onishi H., Nakamura S. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47:421–426. doi: 10.1007/s00535-011-0492-9. [DOI] [PubMed] [Google Scholar]
- 65.Dioguardi Burgio M., Sartoris R., Libotean C., Zappa M., Sibert A., Vilgrain V. Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging. 2019;19:75. doi: 10.1186/s40644-019-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joo I., Kim H.C., Kim G.M., Paeng J.C. Imaging evaluation following (90)Y radioembolization of liver tumors: what radiologists should know. Korean J Radiol. 2018;19:209–222. doi: 10.3348/kjr.2018.19.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh P., Anil G. Yttrium-90 radioembolization of liver tumors: what do the images tell us? Cancer Imaging. 2014;13:645–657. doi: 10.1102/1470-7330.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Veldt A.A., Meijerink M.R., van den Eertwegh A.J., Haanen J.B., Boven E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer. 2010;102:803–809. doi: 10.1038/sj.bjc.6605567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beuzit L., Edeline J., Brun V., Ronot M., Guillygomarc'h A., Boudjema K. Comparison of Choi criteria and Response Evaluation Criteria in Solid Tumors (RECIST) for intrahepatic cholangiocarcinoma treated with glass-microspheres Yttrium-90 selective internal radiation therapy (SIRT) Eur J Radiol. 2016;85:1445–1452. doi: 10.1016/j.ejrad.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 70.Camacho J.C., Kokabi N., Xing M., Prajapati H.J., El-Rayes B., Kim H.S. Modified response evaluation criteria in solid tumors and European Association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol. 2014;25:256–265. doi: 10.1016/j.jvir.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell D.G., Bruix J., Sherman M., Sirlin C.B. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology. 2015;61:1056–1065. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 72.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 73.American College of Radiology Liver imaging reporting and data system. 2018. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS Available at.
- 74.Shropshire E.L., Chaudhry M., Miller C.M., Allen B.C., Bozdogan E., Cardona D.M. LI-RADS treatment response algorithm: performance and diagnostic accuracy. Radiology. 2019;292:182135. doi: 10.1148/radiol.2019182135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seo N., Kim M.S., Park M.-S., Choi J.-Y., Do R.K., Han K. Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with liver imaging reporting and data system version 2017. Eur Radiol. 2020;30:261–271. doi: 10.1007/s00330-019-06376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chun Y.S., Vauthey J.-N., Boonsirikamchai P., Maru D.M., Kopetz S., Palavecino M. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshita H., Hosokawa A., Ueda A., Ando T., Kajiura S., Kato H. Predictive value of optimal morphologic response to first-line chemotherapy in patients with colorectal liver metastases. Digestion. 2014;89:43–48. doi: 10.1159/000356218. [DOI] [PubMed] [Google Scholar]
- 78.Shindoh J., Loyer E.M., Kopetz S., Boonsirikamchai P., Maru D.M., Chun Y.S. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maru D.M., Kopetz S., Boonsirikamchai P., Agarwal A., Chun Y.S., Wang H. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- 80.Mannelli L., Kim S., Hajdu C.H., Babb J.S., Clark T.W., Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009;193:1044–1052. doi: 10.2214/AJR.08.1461. [DOI] [PubMed] [Google Scholar]
- 81.Yuan Z., Zhang J., Yang H., Ye X.D., Xu L.C., Li W.T. Diffusion-weighted MR imaging of hepatocellular carcinoma: current value in clinical evaluation of tumor response to locoregional treatment. J Vasc Interv Radiol. 2016;27:20–30. doi: 10.1016/j.jvir.2015.10.003. quiz 31. [DOI] [PubMed] [Google Scholar]
- 82.Barat M., Fohlen A., Cassinotto C., Jannot A.S., Dautry R., Pelage J.P. One-month apparent diffusion coefficient correlates with response to radiofrequency ablation of hepatocellular carcinoma. J Magn Reson Imaging. 2017;45:1648–1658. doi: 10.1002/jmri.25521. [DOI] [PubMed] [Google Scholar]
- 83.Lu T.-L., Becce F., Bize P., Denys A., Meuli R., Schmidt S. Assessment of liver tumor response by high-field (3 T) MRI after radiofrequency ablation: short-and mid-term evolution of diffusion parameters within the ablation zone. Eur J Radiol. 2012;81:e944–e950. doi: 10.1016/j.ejrad.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Mannelli L., Kim S., Hajdu C.H., Babb J.S., Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur J Radiol. 2013;82:577–582. doi: 10.1016/j.ejrad.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 85.Dong S., Ye X.D., Yuan Z., Xu L.C., Xiao X.S. Relationship of apparent diffusion coefficient to survival for patients with unresectable primary hepatocellular carcinoma after chemoembolization. Eur J Radiol. 2012;81:472–477. doi: 10.1016/j.ejrad.2010.12.081. [DOI] [PubMed] [Google Scholar]
- 86.Kokabi N., Camacho J.C., Xing M., Qiu D., Kitajima H., Mittal P.K. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969–978. doi: 10.1007/s00261-014-0127-8. [DOI] [PubMed] [Google Scholar]
- 87.Chopra S., Dodd G.D., 3rd, Chintapalli K.N., Leyendecker J.R., Karahan O.I., Rhim H. Tumor recurrence after radiofrequency thermal ablation of hepatic tumors: spectrum of findings on dual-phase contrast-enhanced CT. AJR Am J Roentgenol. 2001;177:381–387. doi: 10.2214/ajr.177.2.1770381. [DOI] [PubMed] [Google Scholar]
- 88.Meijerink M.R., van Waesberghe J.H., van der Weide L., van den Tol P., Meijer S., Comans E.F. Early detection of local RFA site recurrence using total liver volume perfusion CT initial experience. Acad Radiol. 2009;16:1215–1222. doi: 10.1016/j.acra.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 89.Choi S.H., Chung J.W., Kim H.C., Baek J.H., Park C.M., Jun S. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010;45:427–436. doi: 10.1097/RLI.0b013e3181e07516. [DOI] [PubMed] [Google Scholar]
- 90.Wang D., Bangash A.K., Rhee T.K., Woloschak G.E., Paunesku T., Salem R. Liver tumors: monitoring embolization in rabbits with VX2 tumors--transcatheter intraarterial first-pass perfusion MR imaging. Radiology. 2007;245:130–139. doi: 10.1148/radiol.2451061689. [DOI] [PubMed] [Google Scholar]
- 91.Braren R., Altomonte J., Settles M., Neff F., Esposito I., Ebert O. Validation of preclinical multiparametric imaging for prediction of necrosis in hepatocellular carcinoma after embolization. J Hepatol. 2011;55:1034–1040. doi: 10.1016/j.jhep.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 92.Gaba R.C., Wang D., Lewandowski R.J., Ryu R.K., Sato K.T., Kulik L.M. Four-dimensional transcatheter intraarterial perfusion MR imaging for monitoring chemoembolization of hepatocellular carcinoma: preliminary results. J Vasc Interv Radiol. 2008;19:1589–1595. doi: 10.1016/j.jvir.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsu C.Y., Shen Y.C., Yu C.W., Hsu C., Hu F.C., Hsu C.H. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55:858–865. doi: 10.1016/j.jhep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 94.Zhu A.X., Sahani D.V., Duda D.G., di Tomaso E., Ancukiewicz M., Catalano O.A. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lassau N., Chapotot L., Benatsou B., Vilgrain V., Kind M., Lacroix J. Standardization of dynamic contrast-enhanced ultrasound for the evaluation of antiangiogenic therapies: the French multicenter Support for Innovative and Expensive Techniques Study. Invest Radiol. 2012;47:711–716. doi: 10.1097/RLI.0b013e31826dc255. [DOI] [PubMed] [Google Scholar]
- 96.Coenegrachts K., Bols A., Haspeslagh M., Rigauts H. Prediction and monitoring of treatment effect using T1-weighted dynamic contrast-enhanced magnetic resonance imaging in colorectal liver metastases: potential of whole tumour ROI and selective ROI analysis. Eur J Radiol. 2012;81:3870–3876. doi: 10.1016/j.ejrad.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 97.De Bruyne S., Van Damme N., Smeets P., Ferdinande L., Ceelen W., Mertens J. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer. 2012;106:1926–1933. doi: 10.1038/bjc.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabet A., Meyer C., Aouf A., Sabet A., Ghamari S., Pieper C.C. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging. 2015;42:370–376. doi: 10.1007/s00259-014-2935-z. [DOI] [PubMed] [Google Scholar]
- 99.Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vernuccio F., Godfrey D., Meyer M., Williamson H.V., Salama J.K., Niedzwiecki D. Local tumor control and patient outcome using stereotactic body radiation therapy for hepatocellular carcinoma: iRECIST as a potential substitute for traditional criteria. AJR Am J Roentgenol. 2019;213:1232–1239. doi: 10.2214/AJR.18.20842. [DOI] [PubMed] [Google Scholar]
- 101.Prasad S.R., Jhaveri K.S., Saini S., Hahn P.F., Halpern E.F., Sumner J.E. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology. 2002;225:416–419. doi: 10.1148/radiol.2252011604. [DOI] [PubMed] [Google Scholar]
- 102.Mantatzis M., Kakolyris S., Amarantidis K., Karayiannakis A., Prassopoulos P. Treatment response classification of liver metastatic disease evaluated on imaging. Are RECIST unidimensional measurements accurate? Eur Radiol. 2009;19:1809–1816. doi: 10.1007/s00330-009-1327-4. [DOI] [PubMed] [Google Scholar]
- 103.Zhao B., James L.P., Moskowitz C.S., Guo P., Ginsberg M.S., Lefkowitz R.A. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–272. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shim J.H., Lee H.C., Kim S.O., Shin Y.M., Kim K.M., Lim Y.S. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 105.Duran R., Chapiro J., Frangakis C., Lin M., Schlachter T.R., Schernthaner R.E. Uveal melanoma metastatic to the liver: the role of quantitative volumetric contrast-enhanced MR imaging in the assessment of early tumor response after transarterial chemoembolization. Transl Oncol. 2014;7:447–455. doi: 10.1016/j.tranon.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin M., Pellerin O., Bhagat N., Rao P.P., Loffroy R., Ardon R. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012;23:1629–1637. doi: 10.1016/j.jvir.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fleckenstein F.N., Schernthaner R.E., Duran R., Sohn J.H., Sahu S., Marshall K. Renal cell carcinoma metastatic to the liver: early response assessment after intraarterial therapy using 3D quantitative tumor enhancement analysis. Transl Oncol. 2016;9:377–383. doi: 10.1016/j.tranon.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bi W.L., Hosny A., Schabath M.B., Giger M.L., Birkbak N.J., Mehrtash A. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69:127–157. doi: 10.3322/caac.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferrante di Ruffano L., Takwoingi Y., Dinnes J., Chuchu N., Bayliss S.E., Davenport C. Computer-assisted diagnosis techniques (dermoscopy and spectroscopy-based) for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013186. doi: 10.1002/14651858.CD013186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leijenaar R.T., Carvalho S., Hoebers F.J., Aerts H.J., van Elmpt W.J., Huang S.H. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol. 2015;54:1423–1429. doi: 10.3109/0284186X.2015.1061214. [DOI] [PubMed] [Google Scholar]
- 111.Lambin P., Rios-Velazquez E., Leijenaar R., Carvalho S., van Stiphout R.G., Granton P. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lambin P., Leijenaar R.T.H., Deist T.M., Peerlings J., de Jong E.E.C., van Timmeren J. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 113.Dohan A., Gallix B., Guiu B., Le Malicot K., Reinhold C., Soyer P. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69:531–539. doi: 10.1136/gutjnl-2018-316407. [DOI] [PubMed] [Google Scholar]
- 114.Brenet Defour L., Mule S., Tenenhaus A., Piardi T., Sommacale D., Hoeffel C. Hepatocellular carcinoma: CT texture analysis as a predictor of survival after surgical resection. Eur Radiol. 2019;29:1231–1239. doi: 10.1007/s00330-018-5679-5. [DOI] [PubMed] [Google Scholar]
- 115.Park H.J., Kim J.H., Choi S.Y., Lee E.S., Park S.J., Byun J.Y. Prediction of therapeutic response of hepatocellular carcinoma to transcatheter arterial chemoembolization based on pretherapeutic dynamic CT and textural findings. AJR Am J Roentgenol. 2017;209:W211–W220. doi: 10.2214/AJR.16.17398. [DOI] [PubMed] [Google Scholar]
- 116.Zhou Y., He L., Huang Y., Chen S., Wu P., Ye W. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:1695–1704. doi: 10.1007/s00261-017-1072-0. [DOI] [PubMed] [Google Scholar]
- 117.Mule S., Thiefin G., Costentin C., Durot C., Rahmouni A., Luciani A. Advanced hepatocellular carcinoma: petreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with sorafenib. Radiology. 2018;288:445–455. doi: 10.1148/radiol.2018171320. [DOI] [PubMed] [Google Scholar]
- 118.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orlhac F., Frouin F., Nioche C., Ayache N., Buvat I. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology. 2019;291:53–59. doi: 10.1148/radiol.2019182023. [DOI] [PubMed] [Google Scholar]
- 120.Graffy P.M., Sandfort V., Summers R.M., Pickhardt P.J. Automated liver fat quantification at nonenhanced abdominal CT for population-based steatosis assessment. Radiology. 2019;293:334–342. doi: 10.1148/radiol.2019190512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morshid A., Elsayes K.M., Khalaf A.M., Elmohr M.M., Yu J., Kaseb A.O. A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization. Radiol Artif Intell. 2019;1:e180021. doi: 10.1148/ryai.2019180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.