Abstract
Background
The United States now has the highest death toll due to COVID‐19. Many otolaryngology procedures, including laryngoscopy, bronchoscopy, and esophagoscopy, place otolaryngologists at increased risk of coronavirus transmission due to close contact with respiratory droplets and aerosolization from the procedure. The aim of this study is to provide an overview of guidelines on how to perform these procedures during the coronavirus pandemic.
Methods
Literature review was performed. Articles citing laryngoscopy, bronchoscopy, esophagoscopy use with regard to COVID‐19 were included.
Results
Laryngoscopy, bronchoscopy, and esophagoscopy are all used in both emergent and elective situations. Understanding the risk stratification of cases and the varied necessity of personal protective equipment is important in protecting patients and health care workers.
Conclusions
Summary guidelines based on the literature available at this time are presented in order to decrease transmission of the virus and protect those involved.
Keywords: bronchoscopy, coronavirus, COVID‐19, esophagoscopy, laryngoscopy
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the virus responsible for COVID‐19 first appeared in Wuhan, China in December 2019. From there it has rapidly spread to become a global pandemic. 1 The United States now has the highest number of deaths due to COVID‐19. 2 The virus is highly infectious with a median daily reproduction rate of 2.35 in Wuhan, China. 3 The most common presenting symptoms of the coronavirus are fever, dry cough, and dyspnea. 1 Although 80% of cases are of asymptomatic to moderate severity, about 6% to 10% of cases progress to require the use of ventilatory support.1, 4
The virus has placed a significant burden on the health care system. Many hospitals are adapting to the new challenges they face in light of SARS‐CoV‐2. A myriad of organizations are creating new guidelines pertaining to COVID‐19 to protect health care workers and decrease the spread of transmission. The virus is transmitted through fomite exposure, respiratory droplets, and aerosolization. Certain procedures, such as bronchoscopy, laryngoscopy, and esophagoscopy, result in close proximity with respiratory droplets and aerosol generation. Thus, the Centers for Disease Control and Prevention have deemed bronchoscopy a high‐risk procedure. 5
Bronchoscopy is used in a variety of diagnostic and therapeutic manners. Specifically in intensive care units, bronchoscopy is valuable in visualizing airways, sampling for diagnostic purposes, and managing artificial airways. 6 In addition to bronchoscopy, laryngoscopy and esophagoscopy may also be used to visualize the airway and remove foreign bodies.7, 8 Due to the high‐risk nature of these procedures, organizations have issued new guidelines to establish safer practices.
The primary aim of this study is to perform a literature review and provide a summary of results of bronchoscopy, laryngoscopy, and endoscopy guidelines with respect to COVID‐19. The second aim of this study is to provide guidelines with the expertise and experience of established otolaryngologists.
2. METHODS
PubMed, Scopus, and Ovid were searched for English‐language articles. The search terms “COVID,” “COVID‐19,” “SARS‐CoV‐2,” “coronavirus,” “bronchoscopy,” “bronchoscope,” “esophagoscopy,” and “laryngoscopy” were used to construct the search function. Literature published by organizations online, which was not peer‐reviewed, was also included in this study. The same search terms used in databases were used in the Google search engine. Guidelines relevant to bronchoscopy, laryngoscopy, and esophagoscopy in regard to COVID‐19 were included. Unpublished documents from the author's affiliated institutions were also reviewed.
3. DISCUSSION
3.1. Diagnosis of COVID‐19
Tracheal aspirate or mini‐bronchoalveolar lavage should be attempted before bronchoscopy is used. 9
Use only if upper respiratory samples are negative and findings would significantly change management.
Example: immunocompromised patient where pneumocystic jirovecii infection must be ruled out.
3.2. Risk stratification of bronchoscopy/laryngoscopy/esophagoscopy cases
Commonly stratified into three categories: emergent, urgent, and nonurgent.10, 11
Emergent cases: moderate‐to‐severe tracheal or bronchial stenosis, symptomatic central airway obstruction, massive hemoptysis, or migrated stent.
Urgent cases: lung mass or mediastinal/hilar lymphadenopathy suspicious for cancer, foreign object aspiration, whole lung lavage, mild/moderate hemoptysis, and suspected infection in immunocompromised patients.
- Nonurgent: all other indications:
- The American Association for Bronchology and Interventional Pulmonology has recommended that all nonurgent cases be postponed until May 2020, at the earliest. 11
Emergent vs urgent cases operating room (OR) pathways (Figure 1).
FIGURE 1.
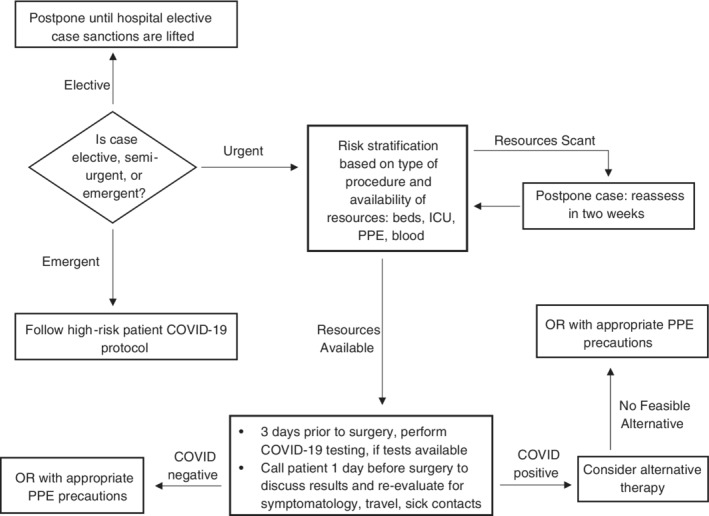
Pathway for operating room cases
3.3. Use of bronchoscopy/esophagoscopy/laryngoscopy in high‐risk COVID‐19 patients
3.3.1. Who?
COVID‐19 testing positive.
COVID‐19 testing pending, but urgent/emergent case.
Has not been tested, but high‐risk factors (symptomatic or contact with COVID‐19 positive individuals).
Emergent case with unknown COVID‐19 status.
3.3.2. Indications
Removal of airway foreign body.
Removal of esophageal foreign body.
Microlaryngoscopy and bronchoscopy (MLB) for airway evaluation of respiratory failure or distress.
MLB for airway dilation.
3.3.3. General considerations
Use proper hand hygiene.
Use personal protective equipment (PPE)—gloves, powered air purifying respirator or N95 respirator mask, face shield, goggles, and fluid repellant gown.
Use extra caution when doffing to prevent contamination.
Use negative pressure room (may not be possible with emergent case).
Ensure oxygen level at safe level (<30%) to prevent fire.
Only essential personnel in the room.
The PPE of each member of the procedural team is detailed in Table 1.
TABLE 1.
Personal protective equipment recommendations for procedure team members
| Anesthesia provider | Scrubing/nursing/scrub PPE | Cleaning crew PPE | |
|---|---|---|---|
| COVID+ patient for any procedure |
|
|
|
| Asymptomatic patient for bronchoscopy, esophagoscopy, laryngoscopy |
|
|
|
| Asymptomatic with negative COVID‐19 testing for any procedure |
|
|
|
Abbreviation: PPE, personal protective equipment.
3.3.4. Surgical site tent
If possible, create surgical tent.
Purpose: will trap particles from being released into the OR environment which is a concern due to aerosolization during otolaryngology procedures.
Equipment for tent: clear plastic drapes: O‐ARM and C‐ARM drapes, smoke evacuator tubing, laryngoscopy suspension arm or ether screen armature, regular procedure equipment.
3.4. Use of bronchoscopy/esophagoscopy/laryngoscopy in low‐risk COVID‐19 patients
Follow OR pathway guidelines in Figure 1.
- If patient tests COVID negative in last 24 to 48 hours.
- Personnel only need standard PPE (Table 1).
- If newly symptomatic, discuss retesting and using more robust PPE.
- If patient is asymptomatic with no recent travel or sick contacts:
- 3 days prior to surgery, call patient in to test them.
- Ask patients to maintain quarantine until surgery.
- Call patients 1 day before surgery and inform them of results and inquire about new onset symptoms, fevers, travel, or contact with COVID‐19 infected persons. 12
- If testing is not available, call 1 day before surgery and ascertain if patient is symptomatic or has had any recent travel/exposure to sick contacts.
- If symptomatic or recent travel or sick contacts, this patient is now high risk.
- In places with high COVID‐19 prevalence, due to the possibility of occult infections, full PPE should still be worn during bronchoscopies if negative. 11
- Detailed use of PPE is detailed in Table 1.
3.5. General recommendations
Using bronchoscopy/laryngoscopy/esophagoscopy for COVID‐19 testing should be last resort.
Always use proper hand hygiene.
Categorize patients based on risk stratification and proceed accordingly.
Use proper PPE based on patient COVID‐19 and risk factors as detailed in Table 1.
Use surgical site tenting when appropriate.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Reddy PD, Nguyen SA, Deschler D. Bronchoscopy, laryngoscopy, and esophagoscopy during the COVID‐19 pandemic. Head & Neck. 2020;42:1634–1637. 10.1002/hed.26221
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center JHCR . Mortality Analysis. Baltimore, MA: Johns Hopkins University; 2020. [Google Scholar]
- 3. Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID‐19: a mathematical modelling study. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diseases NCfIaR . Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID‐19). Atlanta, GA: Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html 2020. [Google Scholar]
- 6. Ergan B, Nava S. The use of bronchoscopy in critically ill patients: considerations and complications. Expert Rev Respir Med. 2018;12(8):651‐663. [DOI] [PubMed] [Google Scholar]
- 7. Mondal PJ, Saha S, Ghosh A, Sengupta M. Removal of foreign bodies from esophagus with flexible endoscope—a case report. Indian J Otolaryngol Head Neck Surg. 2014;66(suppl 1):78‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levitan R, Ochroch EA. Airway management and direct laryngoscopy. A review and update. Crit Care Clin. 2000;16(3):373‐388. v. [DOI] [PubMed] [Google Scholar]
- 9. Anesi GL. Coronavirus Disease 2019 (COVID‐19): critical Care Issues. 2020.
- 10. Medicine UoN . Bronchoscopy Algorithm during COVID‐19. Lincoln, NE: UNMC. https://www.nebraskamed.com/sites/default/files/documents/covid-19/bronchoscopy.pdf. 2020. [Google Scholar]
- 11. Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID‐19 infection. J Bronchol Interv Pulmonol. 2020. 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baldwin DRLW, Rintoul R, Navani N, et al. Recommendations for day case bronchoscopy services during the COVID‐19 pandemic. Eur Assoc Bronchol Interven Pulmonol. 2020. [Google Scholar]


