Summary
Coronavirus disease 2019 (COVID‐19) has had a significant impact on global healthcare services. In an attempt to limit the spread of infection and to preserve healthcare resources, one commonly used strategy has been to postpone elective surgery, whilst maintaining the provision of anaesthetic care for urgent and emergency surgery. General anaesthesia with airway intervention leads to aerosol generation, which increases the risk of COVID‐19 contamination in operating rooms and significantly exposes the healthcare teams to COVID‐19 infection during both tracheal intubation and extubation. Therefore, the provision of regional anaesthesia may be key during this pandemic, as it may reduce the need for general anaesthesia and the associated risk from aerosol‐generating procedures. However, guidelines on the safe performance of regional anaesthesia in light of the COVID‐19 pandemic are limited. The goal of this review is to provide up‐to‐date, evidence‐based recommendations or expert opinion when evidence is limited, for performing regional anaesthesia procedures in patients with suspected or confirmed COVID‐19 infection. These recommendations focus on seven specific domains including: planning of resources and staffing; modifying the clinical environment; preparing equipment, supplies and drugs; selecting appropriate personal protective equipment; providing adequate oxygen therapy; assessing for and safely performing regional anaesthesia procedures; and monitoring during the conduct of anaesthesia and post‐anaesthetic care. Implicit in these recommendations is preserving patient safety whilst protecting healthcare providers from possible exposure.
Keywords: anaesthesia, coronavirus, COVID‐19
Recommendations
1. Planning and preparation
Reduce the clinical load and perform routine testing as per local guidelines. Neuraxial anaesthesia and peripheral nerve blocks are the first choice (whenever possible) for anaesthetic management of patients with suspected COVID‐19 infection.
2. Location
Care of COVID‐19–infected patients should ideally be provided in the operating area and in an airborne infection isolation room if possible. Patients can be operated in a positive pressure room as long as there are measures to prevent airflow from the operating room into the common areas.
3. Personal protective equipment
Regional anaesthesia procedures are not considered aerosol generating, and therefore droplet precautions are recommended as a minimum. Use of a higher level of precautions (against airborne transmission) may be appropriate when caring for patients under spinal anaesthesia in the operating room in certain situations. Patients should wear surgical facemasks to prevent transmission of COVID‐19.
4. Oxygen therapy
The mode of delivery and flow rate of oxygen determines the possibility of aerosol generation and its travelling distance; therefore, the flow of oxygen should be kept to a minimum with the goal to maintain saturation while minimising aerosol generation.
5. Equipment
Minimise the amount of equipment inside the room to what is absolutely essential, and protect the equipment with plastic covers during the procedure.
6. Monitoring and conduct of anaesthesia
Thorough testing for block success is encouraged, to prevent the need for emergency conversion to general anaesthesia. Respiratory monitoring should be ideally performed with the use of viral filters.
7. End of surgery
Patients should be recovered in the operating room or an airborne infection isolation room before being transported to a pre‐designated area.
Introduction
The severe acute respiratory syndrome‐corona virus‐2 (SARS‐CoV‐2) pandemic has reached unprecedented proportions and has significantly impacted healthcare services and surgical volume. Some of the clinical challenges have resulted from the fact that approximately 80% of infected individuals present with no or only mild symptoms of respiratory infection and that, in the absence of universal testing, clinical screening has not allowed the reliable identification of infected patients [1]. In a recent publication, among 210 asymptomatic women admitted for labour and delivery in New York hospitals, 14% had tested positive for SARS‐CoV‐2, emphasising the utility of universal testing in communities with a high prevalence of coronavirus‐2019 (COVID‐19) infection [2].
The virus is highly infectious; the reproductive number (R0), which represents the number of secondary infections resulting from an infected individual, is thought to be 2.6 (95%CI 1.5–3.5) [3]. However, a recent study suggested that the median R0 value of COVID‐19 may be as high as 5.7 [4]. In an attempt to limit the spread of infection and to preserve healthcare resources, including staffing, operating rooms and anaesthesia machines, elective surgical procedures have been postponed in many countries [5]. However, anaesthesia care is still needed for urgent and emergency surgery.
Similar to previous pandemics, healthcare workers are highly vulnerable to contracting the infection. Hence, strategies to minimise exposure and the risk of disease transmission to healthcare workers or patients in the hospital is crucial. Peri‐operative settings and emergency rooms are considered ‘hot zones’ for disease transmission, and measures to minimise exposure and transmission are vital in these areas [6]. One of the strategies to minimise exposure is to avoid aerosol‐generating procedures such as airway management procedures commonly performed in the peri‐operative period. General anaesthesia with airway intervention leads to aerosol generation, which exposes the healthcare team to risk of transmission of COVID‐19 both during tracheal intubation and extubation [7]. The odds of transmission of acute respiratory infection during tracheal intubation to a healthcare worker are thought to be 6.6 times compared with those who are not exposed to tracheal intubation [8]. Tracheal intubation for a COVID‐19–positive patient is ideally performed in a negative pressure room, which may not be available in all places or situations [9]. On the other hand, regional anaesthesia is associated with a lower risk of postoperative complications, and this becomes more important in the context of ongoing respiratory infection [10, 11].
Regional anaesthesia may be the preferred choice for providing anaesthesia care when possible, as it can provide an alternative safe anaesthetic care plan by avoiding the need for aerosol‐generating procedures. Secondly, in the light of expected anaesthetic drug shortages during this pandemic, regional anaesthesia may spare the need for sedatives and hypnotics and hence is less resource‐intensive compared with general anaesthesia. Despite previous respiratory pandemics such as SARS in 2003 and Middle East respiratory syndrome (MERS) in 2012, there is very little evidence‐based guidance available for the practice of regional anaesthesia. An urgent need for such guidelines has been suggested by practising anaesthetists [12].
Our group recently published an interim joint statement by the American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy for the practice of regional anaesthesia during the COVID‐19 pandemic [13]. The current paper aims to provide more detailed, evidence‐based practice recommendations for the safe performance of regional anaesthesia applicable to the current COVID‐19 pandemic.
Methods
A three‐step approach was employed, which included a formal literature search followed by hand searches on individual domains, following which recommendations were generated through mutual consensus. The domains of interest were: planning of resources and staffing; modifying the clinical environment; preparing equipment, supplies and drugs; selecting appropriate personal protective equipment (PPE); providing adequate oxygen therapy; assessing for and safely performing regional anaesthesia procedures; and monitoring during the conduct of anaesthesia and post‐anaesthetic care.
Firstly, a formal literature search was performed for evidence on the use of regional anaesthesia during respiratory pandemics. This was conducted by an experienced librarian (DC) and included PubMed, Embase and the Cochrane Library. The searches were limited to the English language, humans and to a publication date between 1 January 2000 and 9 April 2020. The terms COVID‐19 (or SARS or H1N1 or MERS); anesthesia (or anaesthesia, or anaesthetics or anesthetics); surgery; and/or operating rooms were used for the search (see online Appendix S1). This search aimed to explore all the literature pertinent to the practice of regional anaesthesia during COVID‐19 or similar outbreaks. Specific populations, interventions or outcomes were not added to keep the search broad to include surgical anaesthesia and analgesia.
The titles and abstracts were screened by two authors independently (VU/HK) to select all publications providing recommendations or reporting on the use of regional anaesthesia for a surgical procedure in the context of respiratory infection epidemic caused by viruses similar to SARS‐CoV‐2 (SARS; H1N1; MERS). Any conflicts were resolved by consensus. Backward reference searching was conducted for the selected articles to ensure any essential references were not missed. Full texts of all the selected articles were reviewed in detail, and points relevant to neuraxial and regional anaesthesia were extracted.
Since the formal literature search revealed a paucity of evidence to make any conclusive recommendations, hand searches were performed by the authors to look for either clinical or laboratory evidence on the individual domains relevant to the practice of regional anaesthesia. The literature evidence was further supplemented by professional society guidelines and landmark articles important to the practice of regional anaesthesia. With the collected literature evidence as the basis, practice recommendations were derived through mutual consensus after iterative discussion among the authors.
Results
The literature search identified 987 articles. After title and abstract screening, 16 papers were selected for full‐text review; among those, there was one retrospective cohort study [14]; four case series [15, 16, 17, 18]; four case reports [19, 20, 21, 22]; and seven expert opinion articles [1, 12, 23, 24, 25, 26, 27] (Table 1). Backward citation identified additional reports; however, the information relevant to regional anaesthesia was already presented in original papers in the initial search. An additional case series was identified by a co‐author (RL) during the process of manuscript preparation and was included [28]. The overall quality of evidence was moderate to low, with most studies being single‐centre cohort studies, case‐control studies, case series or case reports. There was very little evidence available from the 17 selected publications regarding oxygen therapy, PPE or the conduct of regional nerve blocks (Fig. 1). Recommendations on these aspects were mainly obtained based on the hand searched articles and society guidelines. The overall quality of evidence was low, and hence the strength of the recommendations is moderate to weak.
Table 1.
Summary of publications reporting on regional anaesthetic or neuraxial procedures in patients with COVID‐19 infection.
| Study | Type | Findings |
|---|---|---|
| Altiparmak et al. [12] | Letter to editor |
Neuraxial anaesthesia and peripheral nerve blocks should be the first choice (whenever possible) for anaesthetic management of patients with suspected COVID‐19 infection. Need for a regional anaesthesia guideline in patients with COVID‐19 infection. |
| Aminnejad et al. [27] | Letter to editor | Debates safety of general anaesthesia vs. neuraxial anaesthesia |
| Bauer et al. [16] | Case series (n = 14) | No reported neurological sequelae after neuraxial procedures in 14 obstetric patients with COVID‐19 infection with varying severity of the infection. Thrombocytopenia was reported in two pregnant patients without pre‐eclampsia. Suggests that the risk of causing meningitis or encephalitis is extremely low with neuraxial procedures, even in infected patients. |
| Bauer et al. [15] | Expert opinion | Early labour epidural analgesia recommended. Maternal hypotension during caesarean delivery with epidural or spinal anaesthesia has not been noted. |
| Breslin et al. [28] | Case series (n = 18) | Eighteen cases with neuraxial anaesthesia in obstetric patients (either using intrapartum epidural analgesia, spinal or combined spinal‐epidural anaesthesia). None had contra‐indications (such as thrombocytopenia or sepsis) to the neuraxial procedure, no haemodynamic instability was noted in any of the patients, and no neurological complications were observed. |
| Chen et al. [17] | Case series (n = 14) | Twelve out of the 14 parturients (86%) undergoing epidural anaesthesia experienced a higher rate of intra‐operative hypotension when 2% lidocaine was used for a loading dose, and 0.75% ropivacaine was used for maintenance. Recommends elective caesarean delivery under neuraxial anaesthesia wherever possible to reduce the possibility of pulmonary complications secondary to intubation. |
| Cohen et al. [25] | Expert opinion | Epidural or paravertebral catheter insertion or epidural blood patch (if indicated) should not be postponed for a COVID‐19 positive patient. |
| Landau et al.[1] | Letter to editor | Pathophysiological changes in pregnancy make interpretation of screening results difficult. Tracheal intubation in one patient was reported to have precipitated immediate, prolonged bronchospasm. Treatment of bronchospasm (nebulisation) could possibly cause aerosolisation of viral particles. |
| Lee et al. [19] |
Case report (for H1N1) |
H1N1 and superimposed bilateral pneumonia. Epidural analgesia for labour followed by vaginal delivery. No complications reported. |
| Lee et al. [20] | Case report | Caesarean delivery; hypotension after spinal anaesthesia stabilised after a few boluses of phenylephrine. The placenta, amniotic fluid and cord blood were all negative for SARS‐CoV‐2 PCR test. |
| Lie, et al. [23] | Expert opinion | The patient should be assessed, the block performed and the patient allowed to recover, inside the operating room where the surgery will be performed to limit contamination to a single location. Consider digital consent to reduce potential paper contamination. The ultrasound machine’s screen and controls protected with a single‐use plastic cover. The CO2 sampling line can be connected to a 15‐mm tracheal tube connector and a high‐efficiency particulate air and heat and moisture exchange filters. Healthcare professional involved in performing regional anaesthesia on a COVID‐19 patient should, at minimum, don PPE, goggles and a surgical facemask. Attempt to minimise diaphragmatic paralysis by modifying the local anaesthetic dose via volume and concentration or the injection site or technique. |
| Maxwell et al. [24] |
Expert opinion (for SARS) |
Neither epidural nor spinal anaesthesia is contra‐indicated. |
| Park et al. [21] |
Case report (for MERS) |
Emergency caesarean delivery for placental abruption. Use of level 3 PPE (airborne precautions) and negative pressure room. |
| Shanthanna, et al. [26] | Expert opinion | The duration of immunosuppression may be shorter with dexamethasone and betamethasone compared with other commonly used steroids used as adjuvants. |
| Xia et al. [22] | Case report | Spinal anaesthesia for emergency caesarean delivery in a patient with moderate to severe COVID‐19 disease. No complications reported. Level 3 PPE (airborne precautions) used |
| Zhao et al. [18] | Case series (n = 11) | Eleven patients received spinal anaesthesia for non‐obstetric surgery. No reported anaesthesia‐related complications. |
| Zhong et al. [14] | Observational cohort study | Spinal anaesthesia for 45 caesarean delivery and four orthopaedic procedures was well tolerated, with no unusual complications. Level 3 PPE (airborne precautions) appear to reduce the risk of transmission to anaesthetists compared with level 1 PPE (contact precaution) |
SARS, severe acute respiratory syndrome; MERS, Middle East respiratory virus; PPE, personal protective equipment; SARS‐CoV‐2, severe acute respiratory syndrome‐coronavirus‐2; PCR, polymerase chain reaction.
Figure 1.
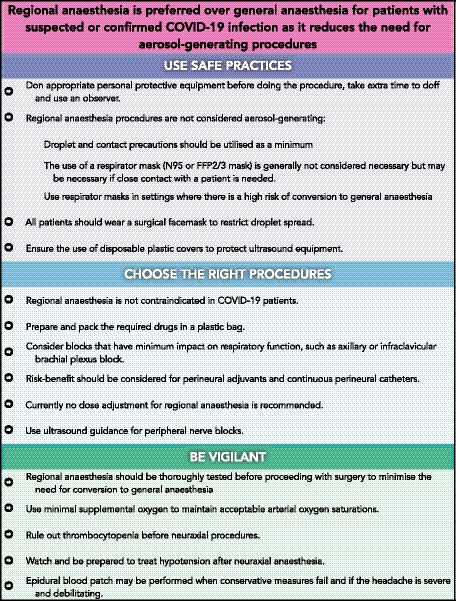
Key recommendations for the performance of regional anaesthesia in suspected or confirmed COVID‐19 patients.
Discussion
The highest available level of evidence relevant to the practice of regional anaesthesia is summarised below under each heading. Although the level of evidence is low for the majority of the above interventions, these recommendations provide a summary of the best available evidence and discuss some uncertainties. The following recommendations apply to a patient with either a suspected or confirmed COVID‐19 infection.
Planning and preparation
Recommendations
Reduce the clinical load and perform routine testing as per local guidelines [29]. Neuraxial anaesthesia and peripheral nerve blocks are the first choice (whenever possible) for anaesthetic management of patients with suspected COVID‐19 infection [12].
Reducing the volume of surgical procedures allows time for institutions to: plan for a surge of patients with COVID‐19; preserve existing stock of PPE; and plan staffing appropriately, particularly as healthcare workers will be quarantined or unwell themselves [30, 31]. This is based on previous governmental regulations implemented during pandemics [32]. All elective operations should be postponed to reduce the risk of exposure of patients and healthcare workers to COVID‐19 and to conserve the capacity of the healthcare system, personnel and resources for a possible increase in demand [29]. Therefore, anaesthesia care should be reserved for urgent and emergent surgery. Guidance for triage of non‐emergent surgical procedures may vary in different countries and may change during the course of the pandemic [33].
Encourage the use of neuraxial anaesthesia and peripheral nerve blocks as the first choice (whenever possible) for anaesthetic management of patients with suspected COVID‐19 infection. Careful consideration should be given to allow the surgery to be performed entirely under regional anaesthesia. An unplanned need for intra‐operative conversion to general anaesthesia is the least desirable outcome. This requires excellent communication between the patient, anaesthetist and the surgical team.
During the COVID‐19 pandemic, preparation for anaesthesia and surgery sometimes entails screening all patients and determining the COVID‐19 status (e.g. COVID‐19 positive, suspected positive (could be under investigation)) [34]. If the community spread is low and a patient is asymptomatic or if tested COVID‐19 negative, then regional anaesthesia can be provided following usual local institutional guidelines as before the pandemic. If the community spread of COVID‐19 infection is significant, all asymptomatic patients should be presumed to be COVID‐19 positive if no testing is being done or while test results are pending.
The possibility of false‐negative results should always be kept in mind. Data have suggested that there is significant variability in the comparative accuracy of different diagnostic modalities, and thus a high index of suspicion and maximising safety procedures is prudent [35]. It is estimated the infection attack rate (the probability of becoming infected) could reach 50–80% of the population, and until evidence suggests that it is safe to presume otherwise, all patients should be presumed to have COVID‐19 infection [36].
Location
Recommendations
Care of COVID‐19–infected patients should ideally be provided in the operating area and in an airborne infection isolation room if possible. Patients can be operated in a positive pressure room as long as there are measures to prevent airflow from the operating room into the common areas.
If available, the care of patients with confirmed or suspected COVID‐19 infection should be provided in a negative pressure room. Nevertheless, surgeries have been safely performed in positive pressure rooms during the SARS outbreak and currently during the COVID‐19 pandemic [37]. There is a theoretical risk of spreading the aerosolised particles to the corridors outside the operating room in a positive pressure room. However, operating rooms have a higher air exchange rate compared with hospital wards or floors. With a standard operating room with a minimum of 15 air exchanges per hour, 99% and 99.9% of airborne contaminants will be removed in 18 min and 28 min, respectively [38]. Alternatively, another report suggests decreasing the inflow while increasing the exhaust can enable the room to remain at neutral pressure while still maintaining laminar flow over the surgical area [37].
The regional anaesthesia procedure for a patient with suspected or confirmed COVID‐19 infection should be performed in the operating room, or a labour room for an obstetric patient. The use of common areas, such as a block room or a holding area, should be avoided to reduce the risk of cross infection. If possible, record keeping or electronic recordings should be done outside the room.
Personal protective equipment
Recommendations
Regional anaesthesia procedures are not considered aerosol‐generating, and therefore droplets precautions are recommended as a minimum (Fig. 2 ). Use of a higher level of precautions (airborne precautions) may be appropriate when caring for patients under spinal anaesthesia in the operating room in certain situations (Fig. 2 ). Patients should wear surgical facemasks to prevent transmission of COVID‐19.
Figure 2.
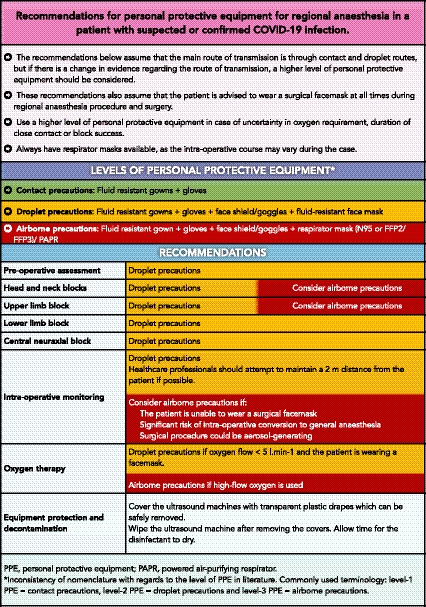
Recommendations for personal protective equipment for regional anaesthesia in a patient with suspected or confirmed COVID‐19 infection.
Protection of healthcare workers when caring for a patient during the COVID‐19 pandemic necessitates appropriate PPE, especially in light of PPE shortages. An appropriate level of PPE is determined by both the medical procedure and the proximity of a healthcare worker to the patient. Personal protective equipment can be classified into three levels (Fig. 2): contact precautions; droplet precautions; and airborne precautions [39]. A healthcare worker caring for a suspected or confirmed COVID‐19 patient within 2 m should use droplet precautions if the procedure is not an aerosol‐generating procedure.
In a small, retrospective, cohort study, airborne precautions reduced the transmission risk to anaesthetists exposed to mildly symptomatic surgical patients during spinal anaesthesia when compared with contact precautions [14]. Of importance, neither neuraxial anaesthesia nor peripheral nerve blocks are considered to be aerosol‐generating procedures; therefore, applying regular contact and droplet precautions for these low‐risk procedures suffice [40]. Personal protective equipment includes a surgical mask, eye protection (goggles or face shield), an impervious surgical gown, and gloves for personnel involved in performing these procedures. The use of respirator masks (e.g. N95 or FFP2/3) is not generally needed but may be considered for prolonged close contact with a COVID‐19–infected patient in a closed environment [41].
Keeping this in mind, if the chances of intra‐operative conversion to general anaesthesia are significant with the need for airway intervention (an aerosol‐generating procedure), it may be appropriate to use a respirator mask. The anticipated urgency and probability of conversion to general anaesthesia is an essential factor when making the decision to wear a respirator mask. Importantly, all patients should wear a surgical mask to restrict the droplet spread [42]. The most experienced person should perform the regional anaesthesia technique. The donning of PPE should occur before entering the room. The presence of an observer during the donning and doffing procedure is highly recommended. Simulation sessions should be conducted for training staff in donning and doffing of PPE [34].
Much confusion exists among providers as to: what the levels of PPE mean; whether the route of transmission is airborne or droplets and contact; and what is the difference between aerosol vs. droplet vs. droplet nuclei vs. airborne. Some of these concepts have been clarified in a recent review [39]. Such controversies existed in previous pandemics too. Several independent risk factors have shown to be responsible for nosocomial transmission of SARS, and the same may hold true for COVID‐19. These include: a distance between beds of less than 1 m; resuscitation attempts; presence of symptomatic caregivers; the need for oxygen therapy; or non‐invasive positive pressure ventilation. While many of these domains may not be relevant to the practice of regional anaesthesia, oxygen therapy needs consideration.
Oxygen therapy
Recommendations
The mode of delivery and flow rate of oxygen determines the possibility of aerosol generation and its travelling distance, therefore; the flow of oxygen should be kept to a minimum with the goal to maintain saturation while minimising aerosol generation.
Oxygen supplementation can result in exhaled air jets and may result in droplet nuclei formation. Whether or not this may result in respirable infectious aerosols depends on a variety of factors, such as: the type of oxygen therapy utilised; the viral load in each breath; ventilation and air exchange in the room; and the use of facemask by the patient, among others. The type of oxygen therapy determines the travelling distances of the exhaled air with the least distance (0.4 m) seen with the use of a Hudson mask utilising 4 l.min−1 of oxygen flow [43], followed by nasal cannula 1 m caudally with the use of 5 l.min−1 of oxygen flow [44] and probably the maximum by a jet nebuliser (> 0.8 m laterally) when using 6 l.min−1 oxygen flows [45].
The presence of an exhaled jet does not necessarily translate into the presence of respirable droplet nuclei or aerosols, as these studies have been conducted using smoke plumes rather than actual detection of infectious aerosols. Also, the concentration of airborne particles is known to decrease over distance, irrespective of other factors such as airflow [46]. It is a common practice to protect the mouth and nose of patients with respiratory infection as this may reduce person‐to‐person transmission of respiratory infectious viruses. Surgical masks seem to be as effective as respirator masks in decreasing transmission of nosocomial and healthcare worker infections [47].
Based on the above evidence, the use of high oxygen flows through nasal cannulae should be avoided to reduce the risk of possible aerosol generation [48]. If the patient needs supplemental oxygen, an oxygen mask should be preferred over nasal prongs. The flow of supplemental oxygen should be kept to the minimum (preferably < 5 l.min−1) needed to maintain arterial oxygen saturation to reduce the risk of aerosolisation [49]. Surgical facemasks can be used over oxygen masks to limit the dispersion of droplets.
Equipment
Recommendation
Minimise the amount of equipment inside the room to what is absolutely essential, and protect the equipment with plastic covers during the procedure.
The required equipment and drugs for immediate peri‐operative care should be prepared and packed in a plastic bag [50]. The ultrasound equipment, including an ultrasound transducer, should be protected from contamination using plastic covers [23]. Handheld ultrasound devices are preferable to larger units for suspected or confirmed COVID‐19 patients. If using trolley‐based ultrasound machines, extra attachments such as baskets and printers should be removed. Single‐use ultrasound gel packs are preferable over multi‐use gel bottles. Bringing a cart or trolley with drugs and equipment to the procedure room should be discouraged. The number of personnel present during the performance of the procedure should be minimised, but help (e.g. a ‘runner’) should be readily available.
There is evidence demonstrating that COVID‐19 virus particles could remain viable on plastic for up to 3 days [51]. However, as most available disinfectants are effective against SARS‐CoV‐2, it is recommended that the ultrasound machine be wiped twice, once inside the room and then again outside the room. Adequate time should be given to allow the surface to dry after each wipe [52]. Institutional protocols should be used for decontamination of equipment.
Neuraxial procedures
Recommendations
Presence of COVID‐19 infection in itself is not a contra‐indication to performing neuraxial anaesthesia. It is advisable to rule out thrombocytopenia before attempting neuraxial techniques in a suspected or confirmed COVID‐19 patient.
Management of obstetric patients with suspected or confirmed COVID‐19 during labour and delivery requires specific considerations [21]. The crucial physiological difference and urgency should be taken into consideration when making decisions regarding neuraxial procedures in pregnant women. Assessment of the suitability of neuraxial procedures requires balancing the risks of general anaesthesia. A factor that should be taken into consideration is the presence of any pre‐operative respiratory compromise that may deteriorate due to further loss of functional residual capacity after neuraxial anaesthesia.
Preliminary evidence suggests that thrombocytopenia might occur in patients with severe COVID‐19 disease [53], while other data demonstrate lower platelet counts in COVID‐19 positive patients compared with COVID‐19 negative patients [54, 55]. It is therefore advisable to rule out thrombocytopenia before attempting neuraxial techniques in a COVID‐19–positive or suspected patient. A platelet count of 75,000 × 106.l−1 or above has been suggested as an acceptable level for performing of neuraxial procedures in the obstetric population, provided the platelet function is expected to be normal [56].
The use of neuraxial procedures during pregnancy and delivery was reported in early studies from China. A recent review described 14 cases of neuraxial anaesthesia for delivery pooled from four different reports [16]. Fever was reported in all patients, but none had a high white cell count, except one that was attributed to the concomitant use of methylprednisolone for unrelated inflammatory conditions. In general, the presence of COVID‐19 infection in the absence of any other risk factors or laboratory abnormality is not a contra‐indication for neuraxial procedures in obstetric or non‐obstetric patients with COVID‐19 infection [18, 22]. Routine indications and contra‐indications for neuraxial anaesthesia apply when managing suspected or confirmed COVID‐19 patients.
In a cohort of obstetric patients in New York, tested positive for COVID‐19 infection, among 18 consecutive women receiving neuraxial labour analgesia or anaesthesia for caesarean delivery, none had a contra‐indication (e.g. thrombocytopenia or sepsis) for a neuraxial procedure, no haemodynamic instability was reported during the surgery, and no neurological complications were noted in the postpartum period [28].
Caution should be exercised when attempting to reduce the duration of the spinal anaesthetic by using short‐acting spinal anaesthetics or reducing the dose of the spinal anaesthetic agent, as conversion to general anaesthesia is the least desirable outcome. Routine asepsis techniques being practised for non‐COVID patients should still be followed. If available, an epidural positioning device could be used to reduce the contact of the assistant with a suspected or confirmed COVID‐19 patient. Currently, no dose adjustment for spinal anaesthesia or adjuvant opioids is recommended. However, a change to the epidural infusion regimen may be needed to reduce the need for additional top‐up doses that require frequent patient contact.
A single, small case series suggested the possibility of excessive intra‐operative hypotension when prophylactic vasopressors were not used [17]; however, hypotension following neuraxial anaesthesia has not otherwise been reported. Anaesthetists should be prepared to manage hypotension following neuraxial procedures as for any other patient [57, 58].
Management of post‐dural puncture headache
Recommendations
As with usual care, pharmacological approaches should be proposed before performing an epidural blood patch. Complications should be discussed on a case‐by‐case basis.
There are currently no available data to guide the management of post‐dural puncture headache (PDPH) in patients with COVID‐19 infection. Pharmacological approaches should be proposed as with any other patient. Concern about the injection of viraemic blood into the epidural space with an epidural blood patch has been raised, especially during active illness, although there is currently no evidence to suggest that this may be the case. If the PDPH is severe and debilitating, an epidural blood patch should be proposed, balancing the risk of neurological complications associated with severe untreated PDPH against the theoretical risk associated with the injection of possibly viraemic blood in the epidural space [25].
Nasal sphenopalatine ganglion block might be an aerosol‐generating procedure, as it involves an injection/insertion into the nasal cavity, and may increase the risk of COVID‐19 transmission to healthcare workers. Therefore, it should be avoided in patients with suspected or confirmed COVID‐19.
Peripheral nerve block
Recommendations
If performing a peripheral nerve block near the head and neck area, in addition to droplet precautions, precautions against airborne virus transmission may be considered. Use ultrasound guidance to reduce the risk of local anaesthetic systemic toxicity.
The evidence regarding the use of peripheral nerve blocks is minimal. Recently, Lie et al. published practical considerations for performing regional anaesthesia, and the American and European Societies of Regional Anaesthesia have published a practice recommendation on the topic [13, 23]. In general, peripheral nerve blocks are considered to result in fewer physiological consequences or haemodynamic side‐effects compared with neuraxial techniques. Most peripheral nerve blocks do not cause sympathectomy leading to hypotension. In terms of the risk of haematoma, a few deep peripheral nerve blocks are considered similar but still are less likely to cause compressive symptoms as the peripheral nerves are not enclosed in a spinal canal.
Patient preparation and asepsis should be similar to that followed for the neuraxial procedure. If possible, attempts should be made to choose the block that is least likely to interfere with respiratory function. In other words, axillary or infraclavicular brachial plexus block may be chosen over supraclavicular brachial plexus block, and superior trunk block or other alternatives are preferred over interscalene block.
The dose of pre‐procedural sedation, if used, may need to be reduced to avoid any respiratory compromise requiring supplemental oxygen. A safe dose of local anaesthetics should be calculated and used. Blocks should be performed with ultrasound guidance to reduce the risk of local anaesthetic systemic toxicity [59]. The benefit of perineural adjuvants must be balanced against the risks of immunosuppression (dexamethasone); sedation; bradycardia and hypotension (clonidine and dexmedetomidine); drug errors; and drug contamination [26].
The decision to insert and maintain perineural catheters needs to be made on a case‐by‐case basis. While continuous catheter techniques can be labour‐ and resource‐intensive and may require frequent patient contact, the opioid‐sparing effect of regional anaesthesia can be beneficial to a patient with respiratory morbidity. Hence, the use of inpatient perineural catheters should be evaluated based on patient needs and available resources. Ambulatory perineural catheters may still be utilised with clear patient instructions.
Similarly, the risk‐benefit ratio of analgesic peripheral nerve blocks and fascial plane blocks should be evaluated on a case‐by‐case basis. If the block is performed under general anaesthesia and requires repositioning of the patient, there is a risk of tracheal tube disconnection or dislodgement. Therefore, it may be advisable to choose a block that does not require patient repositioning (e.g. transversus abdominis plane blocks) over those that require repositioning (e.g. erector spinae plane block), if appropriate. In general, any additional analgesic block procedures should be avoided if adequate analgesia can be achieved using alternate regimens such as systemic analgesia. If the patient is not wearing a surgical mask during an upper limb procedure, the patient can be requested to turn the head away from the operator or plastic drapes can be used to limit droplet spread to the anaesthetist.
Monitoring and conduct of anaesthesia
Recommendations
Thorough testing for block success is encouraged to prevent the need for emergent conversion to general anaesthesia. Respiratory monitoring should be ideally performed with the use of viral filters.
Both neuraxial anaesthesia and peripheral nerve block should be thoroughly tested for block success before proceeding with surgery to minimise the risk of conversion to general anaesthesia. In the case of peripheral nerve block, extra onset time should be allowed to reduce the risk of conversion. If intra‐operative conversion to general anaesthesia is required, the emergency airway procedure should be followed, as described in the literature [49]. Excessive or deep sedation should be avoided to reduce the need for any airway manipulation or interventions.
Our current understanding of COVID‐19 spread suggests that coughing and sneezing lead to the generation of droplets. However, the patient should wear a surgical facemask at all times throughout the procedure. Prolonged close contact with a suspected or confirmed COVID‐19 patient should be avoided wherefore possible.
There are concerns about anaesthetic machine contamination by the virus via end‐tidal carbon dioxide monitoring, as the carbon dioxide sample line draws the gas sample directly without passing through a heat and moisture exchange filter. The contaminated gases generally enter the gas analyser through a water trap. The water trap of the anaesthesia machines includes a filter that typically filters approximately 99.999% of viruses. The filtering capacity of each filter can be obtained from the instruction manual provided by the manufacturer. However, the disposal of the water trap filter is recommended after use for an infected patient. Alternatively, use of a 0.2‐micron membrane filter (Fig. 3), epidural filter or heat‐moisture exchange filters can potentially allow the re‐use of the water trap between the patients [23, 60].
Figure 3.

Possible arrangements to allow the re‐use of capnography sampling tubing between patients. (a) A membrane filter between the CO2 line and water trap. (b) A membrane filter connected to the CO2 line at the mask end of tubing.
End of surgery
Recommendations
Patients should be recovered in the operating room or an airborne infection isolation room before being transported to a pre‐designated area.
The patient should be monitored in the operating room until safe and before transfer to a COVID‐19 designated area of the hospital, in accordance with local guidelines. It has been shown that the risk of transmission is highest during the doffing of PPE, therefore extra time should be allowed for donning and doffing [61, 62]. Any re‐usable equipment utilised during the procedure should be disinfected as per institutional guidelines.
Limitations of the review and future directions
The dearth of robust evidence precludes making any strong practice recommendations, despite the COVID‐19 pandemic not being the first respiratory pandemic in the last two decades. Current evidence has been generated through individual case series or a retrospective cohort of cases from single institutions. National and societal registries will provide additional data to guide safe practices in the coming months [63, 64]. Individual experiences are vital in formulating treatment plans in the light of an epidemic, and a similar learning lesson was seen during the SARS outbreak when the contributions of the frontline workers and a grounded theory approach helped in formulating a risk management framework and management guidelines for the safe performance of aerosol‐generating procedures [65]. A similar effort is needed to generate evidence‐based practice recommendations in regional anaesthesia. Future evidence on the disease course may change our recommendations.
Supporting information
Appendix S1. Search strategy.
Acknowledgements
We thank D. Chapman (DC), Manager, Library Services, IWK Health Centre (Halifax), for helping us with the literature search. KE is an Editor of Anaesthesia and has received research or educational funding from GE Healthcare, Ambu and Fisher and Paykel Healthcare Ltd. No other external funding or competing interests declared.
Contributor Information
V. Uppal, Email: v.uppal@dal.ca, @ropivacaine.
R. V. Sondekoppam, @rakesh6282.
R. Landau, @ruthi_landau.
K. El‐Boghdadly, @elboghdadly.
S. Narouze, @NarouzeMD.
H. K. P. Kalagara, @KalagaraHari.
References
- 1. Landau R, Bernstein K, Mhyre J. Lessons learned from first COVID‐19 cases in the United States. Anesthesia and Analgesia 2020. Epub 31 March. 10.1213/ANE.0000000000004840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. New England Journal of Medicine 2020; 382:2163‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imperial College London COVID‐19 Response Team . Report 3: Transmissibility of 2019‐nCoV. 2020. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-transmissibility-25-01-2020.pdf (accessed 13/04/2020).
- 4. Sanche S, Lin YT, Xu C, Romero‐Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases 2020; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stahel PF. How to risk‐stratify elective surgery during the COVID‐19 pandemic? Patient Safety in Surgery 2020; 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz J, King CC, Yen MY. Protecting health care workers during the COVID‐19 coronavirus outbreak ‐lessons from Taiwan's SARS response. Clinical Infectious Diseases 2020. Epub 12 March. 10.1093/cid/ciaa255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Infection prevention and control of epidemic‐and pandemic‐prone acute respiratory diseases in health care. 2007. https://www.who.int/csr/resources/publications/WHO_CD_EPR_2007_6/en (accessed 25/04/2020). [PubMed]
- 8. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019‐nCoV) patients. Canadian Journal of Anesthesia 2020; 67: 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren J, Sundaram K, Anis H, et al. Spinal anesthesia is associated with decreased complications after total knee and hip arthroplasty. Journal of the American Academy of Orthopaedic Surgeons 2020; 28: e213–e221. [DOI] [PubMed] [Google Scholar]
- 11. von Ungern‐Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: a prospective cohort study. Lancet 2010; 376: 773–83. [DOI] [PubMed] [Google Scholar]
- 12. Altiparmak B, Korkmaz Toker M, Uysal AI, Gümüş Demïrbïlek S. Regional anesthesia in patients with suspected COVID‐19 infection. Regional Anesthesia & Pain Medicine 2020. Epub 3 April. 10.1136/rapm-2020-101477 [DOI] [PubMed] [Google Scholar]
- 13. Uppal V, Sondekoppam RV, Lobo CA, Kolli S, Kalagara HKP. Practice recommendations on neuraxial anesthesia and peripheral nerve blocks during the COVID‐19 pandemic. https://www.asra.com/page/2905/practice-recommendations-on-neuraxial-anesthesia-and-peripheral-nerve-blocks-dur (accessed 11/04/2020). [DOI] [PMC free article] [PubMed]
- 14. Zhong Q, Liu YY, Luo Q, et al. Spinal anaesthesia for patients with coronavirus disease 2019 and possible transmission rates in anaesthetists: retrospective, single‐centre, observational cohort study. British Journal of Anaesthesia 2020; 124:670‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauer M, Bernstein K, Dinges E, et al. Obstetric anesthesia during the COVID‐19 pandemic. Anesthesia & Analgesia 2020; 131:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauer ME, Chiware R, Pancaro C. Neuraxial procedures in COVID‐19 positive parturients: a review of current reports. Anesthesia and Analgesia 2020. Epub 26 March. 10.1213/ANE.0000000000004831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing Cesarean delivery: a case series of 17 patients. Canadian Journal of Anesthesia 2020; 67:655‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao S, Ling K, Yan H, et al. Anesthetic management of patients with COVID 19 Infections during emergency procedures. Journal of Cardiothoracic and Vascular Anesthesia 2020; 34: 1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee AI, Hoffman MJ, Allen NN, Sullivan JT. Neuraxial labor analgesia in an obese parturient with influenza A H1N1. International Journal of Obstetric Anesthesia 2010; 19: 223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DH, Lee J, Kim E, Woo K, Park HY, An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS‐ CoV‐2) confirmed patient. Korean Journal of Anesthesiology 2020; 73:347‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park MH, Kim HR, Choi DH, Sung JH, Kim JH. Emergency cesarean section in an epidemic of the Middle East respiratory syndrome: a case report. Korean Journal of Anesthesiology 2016; 69: 287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia H, Zhao S, Wu Z, Luo H, Zhou C, Chen X. Emergency Caesarean delivery in a patient with confirmed coronavirus disease 2019 under spinal anaesthesia. British Journal of Anaesthesia 2020; 124:e216‐e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lie SA, Wong SW, Wong LT, Wong TGL, Chong SY. Practical considerations for performing regional anesthesia: lessons learned from the COVID‐19 pandemic. Canadian Journal of Anesthesia 2020. Epub 24 March. 10.1007/s12630-020-01637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maxwell C, McGeer A, Tai KFY, et al. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). Journal of Obstetrics and Gynaecology Canada 2009; 31: 358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen SP, Baber ZB, Buvanendran A, et al. Pain management best practices from multispecialty organizations during the COVID‐19 pandemic and public health crises. Pain Medicine 2020; 21:1331‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shanthanna H, Strand NH, Provenzano DA, et al. Caring for patients with pain during the COVID‐19 pandemic: consensus recommendations from an international expert panel. Anaesthesia 2020; 75:935‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aminnejad R, Shafiee H. Is regional anesthesia safe enough in suspected or confirmed COVID‐19 patients? ACS Chemical Neuroscience 2020; 11:1371. [DOI] [PubMed] [Google Scholar]
- 28. Breslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American Journal of Obstetrics and Gynecology MFM 2020; 2:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Besnier E, Tuech JJ, Schwarz L. We asked the experts: Covid‐19 outbreak: is there still a place for scheduled surgery? “reflection from pathophysiological data”. World Journal of Surgery 2020; 44(6): 1695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lancaster EM, Sosa JA, Sammann A, et al. Rapid response of an academic surgical department to the COVID‐19 pandemic: implications for patients, surgeons, and the community. Journal of the American College of Surgeons 2020; 230:1064‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorbello M, El‐Boghdadly K, Di Giacinto I, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia 2020; 75:724‐32. [DOI] [PubMed] [Google Scholar]
- 32. Oberholtzer K, Sivitz L, Mack A, Lemon S, Mahmoud A, Knobler S. Learning from SARS: preparing for the next disease outbreak: workshop summary: National Academies Press. 2004. https://www.ncbi.nlm.nih.gov/books/NBK92462 (accessed 25/04/2020). [PubMed]
- 33. American College of Surgeons . COVID‐19: guidance for triage of non‐emergent surgical procedures. 2020. https://www.facs.org/covid-19/clinical-guidance/triage (accessed 18/04/2020).
- 34. Wong J, Goh QY, Tan Z, et al. Preparing for a COVID‐19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Canadian Journal of Anesthesia 2020; 67:732‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. Journal of the American Medical Association 2020; 323:1843‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infectious Diseases 2020. Epub 30 March. 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu EH, Koh KF, Chen FG. Outbreak of severe acute respiratory syndrome in Singapore and modifications in the anesthesia service. Anesthesiology 2004; 100: 1629–30. [DOI] [PubMed] [Google Scholar]
- 38. Centres for Disease Prevention . Guidelines for environmental infection control in health‐care facilities. 2003. https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html#tableb2 (accessed 14/04/2020).
- 39. Cook TM. Personal protective equipment during the COVID‐19 pandemic ‐ a narrative review. Anaesthesia 2020; 75:920‐27. [DOI] [PubMed] [Google Scholar]
- 40. Association of Anaesthetists . Personal Protective Equipment (PPE) for clinicians. 2020. https://icmanaesthesiacovid-19.org/personal-protective-equipment-ppe-for-clinicians (accessed 30/03/2020).
- 41. World Health Organization . Rational use of personal protective equipment for coronavirus disease 2019 (COVID‐19). 2020. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf (accessed 30/03/2020).
- 42. World Health Organization . Coronavirus disease (COVID‐19) advice for the public: when and how to use masks. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (accessed 30/03/2020).
- 43. Hui DS, Ip M, Tang JW, et al. Airflows around oxygen masks: a potential source of infection? Chest 2006; 130: 822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hui DS, Chow BK, Chu L, et al. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology 2011; 16: 1005–13. [DOI] [PubMed] [Google Scholar]
- 45. Hui DS, Chow BK, Chu LCY, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 2009; 135: 648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peters CJ, Jahrling PB, Khan AS. Patients infected with high‐hazard viruses: scientific basis for infection control. Archives of Virology. Supplementum 1996; 11: 141–68. [DOI] [PubMed] [Google Scholar]
- 47. Long Y, Hu T, Liu L, et al. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta‐analysis. Journal of Evidence‐Based Medicine 2020; 13:93‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lyons C, Callaghan M. The use of high‐flow nasal oxygen in COVID‐19. Anaesthesia 2020; 75:843‐47. [DOI] [PubMed] [Google Scholar]
- 49. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐ 19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020; 75:785‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matava CT, Yu J, Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID‐19. Canadian Journal of Anesthesia 2020; 67:902‐04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine 2020; 382:1564‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim DJ, Jelic T, Woo MY, Heslop C, Olszynski P. Just the facts: recommendations on point of care ultrasound use and machine infection control during the COVID‐19 pandemic. Canadian Journal of Emergency Medicine 2020. Epub 9 April. 10.1017/cem.2020.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clinica Chimica Acta 2020; 506: 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine 2020; 8:475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bauer ME, Toledano RD, Houle T, et al. Lumbar neuraxial procedures in thrombocytopenic patients across populations: a systematic review and meta‐analysis. Journal of Clinical Anesthesia 2020; 61: 109666. [DOI] [PubMed] [Google Scholar]
- 57. Uppal V, McKeen DM. Strategies for prevention of spinal‐associated hypotension during Cesarean delivery: are we paying attention? Canadian Journal of Anesthesia 2017; 64: 991–6. [DOI] [PubMed] [Google Scholar]
- 58. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018; 73: 71–92. [DOI] [PubMed] [Google Scholar]
- 59. El‐Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local and Regional Anesthesia 2018; 11: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anesthesia Patient Safety Foundation . FAQ on anesthesia machine use, protection, and decontamination during the covid‐19 pandemic. 2020. https://www.apsf.org/faq-on-anesthesia-machine-use-protection-and-decontamination-during-the-covid-19-pandemic/#gas (accessed 11/04/2020).
- 61. Chughtai AA, Chen X, Macintyre CR. Risk of self‐contamination during doffing of personal protective equipment. American Journal of Infection Control 2018; 46: 1329–34. [DOI] [PubMed] [Google Scholar]
- 62. Lim SM, Cha WC, Chae MK, Jo IJ. Contamination during doffing of personal protective equipment by healthcare providers. Clinical and Experimental Emergency Medicine 2015; 2: 162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Obstetric Anaesthetists' Association . ObsCOVID. 2020. https://obscovid.org (accessed 18/04/2020).
- 64. Difficult Airway Society . intubateCOVID. 2020. https://intubatecovid.org (accessed 18/04/2020).
- 65. Nanji KC, Orser BA. Managing Ebola: lessons learned from the SARS epidemic. Anesthesia and Analgesia 2015; 121: 834–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.


