Abstract
The current coronavirus disease 2019 (COVID-19) pandemic requires extra attention for immunocompromised patients, including solid organ transplant recipients. We report on a case of a 35-year-old renal transplant recipient who suffered from a severe COVID-19 pneumonia. The clinical course was complicated by extreme overexposure to the mammalian target of rapamycin inhibitor everolimus, following coadministration of chloroquine and lopinavir/ritonavir therapy. The case is illustrative for dilemmas that transplant professionals may face in the absence of evidence-based COVID-19 therapy and concurrent pressure for exploration of experimental pharmacological treatment options. However, the risk-benefit balance of experimental or off-label therapy may be weighed differently in organ transplant recipients than in otherwise healthy COVID-19 patients, owing to their immunocompromised status and potential drug interactions with immunosuppressive therapy. With this case report, we aimed to achieve increased awareness and improved management of drug-drug interactions associated with the various treatment options for COVID-19 in renal transplant patients.
KEYWORDS: clinical decision-making, clinical research/practice, drug interaction, immunosuppressive regimens, infection and infectious agents – viral, infectious disease, kidney transplantation/nephrology, pharmacokinetics/pharmacodynamics, pharmacology
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; CYP, cytochrome p450 enzyme; FDA, US Food and Drug Administration; FiO2, fraction inspired oxygen; ICU, intensive care unit; MERS, Middle East respiratory syndrome; mTORi, mammalian target of rapamycin inhibitor; NSAID, non-steroidal anti-inflammatory drug; PEEP, positive end expiratory pressure; P-gp, p-glycoprotein; RIVM, Rijksinstituut voor Volksgezondheid en Milieu; SARS-CoV, severe acute respiratory syndrome coronavirus; TDM, therapeutic drug monitoring; UGT, uridine diphosphate glucuronosyltransferase enzyme
1. INTRODUCTION
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak has developed rapidly into a global pandemic. Most (inter)national outbreak management organizations consider organ transplant recipients a risk group for COVID-19 complications because of comorbidity and vulnerability from maintenance immunosuppression. Reports of solid organ transplant recipients suffering from respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses suggested poor outcome.1 Contrarily, this was not seen for Middle East respiratory syndrome (MERS).2 It is currently unknown whether renal transplant recipients have increased susceptibility to develop complications of COVID-19 as compared to nontransplanted patients with end-stage renal disease. Previous reports suggested that immunosuppression might diminish the “immune system overdrive” underlying the development of acute respiratory distress syndrome (ARDS) and subsequent mortality.3 Moreover, some immunosuppressants are hypothesized to have antiviral properties. Cyclosporine has shown in vitro antiviral potency against SARS-CoV-1 via cyclophilin inhibition,4 whereas mycophenolate and mammalian target of rapamycin inhibitors (mTORis) may affect MERS-CoV (in vitro and in vivo) and SARS-CoV-1 (in vitro).2 The exact biological mechanisms are, however, unknown.
Currently, no evidence-based treatment for COVID-19 is available, with recommendations merely supported by in vitro studies. The Dutch Center for Disease Control and Prevention (Rijksinstituut voor Volksgezondheid en Milieu; RIVM) suggested initially chloroquine and lopinavir/ritonavir as an option for hospitalized patients based on the MERS and SARS-CoV-1 outbreaks.5, 6, 7
Given the current pressure for experimental treatment for COVID-19, potential drug-drug interactions may befall organ transplant recipients using immunosuppression.
We report on a renal transplant recipient with highly elevated everolimus concentrations after initiation of chloroquine and lopinavir/ritonavir for COVID-19 pneumonia. The main goal of this case report is to raise awareness for pharmacokinetic interactions between experimental COVID-19 therapy and maintenance immunosuppression in this particularly vulnerable population.
2. CASE
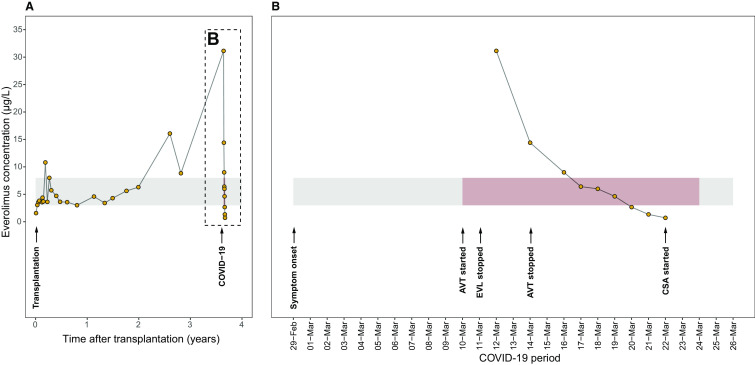
The patient is a 35-year-old male with a medical history of end-stage renal disease of unknown origin. He underwent living-related renal transplantation in 2016. Immunosuppressive therapy was initiated according to the Triton study protocol (NCT02057965), which included subcutaneous alemtuzumab induction (15 mg QD on 2 consecutive days) and autologous mesenchymal stromal cells. Since transplantation, he has been receiving dual maintenance immunosuppressive therapy with everolimus (3 mg BID) and prednisolone (7.5 mg QD), with a stable serum creatinine of around 1.8 mg/dL, an estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration) of 49 mL/min/1.73m2 without albuminuria. Everolimus was managed by therapeutic drug monitoring (TDM), targeted at trough concentrations between 3-8 µg/L. The course of everolimus TDM since transplantation and timing of essential aspects in this case are illustrated in Figure 1A.
FIGURE 1.
Schematic depiction of the course of everolimus concentrations since (A) the transplantation procedure and (B) the onset of coronavirus disease 2019 (COVID-19)–related symptoms. The gray-shaded area depicts the everolimus trough target range, with the period of hospital admission indicated in red. The timing of essential aspects of this case report are indicated with arrows. COVID-19, coronavirus disease 2019; AVT, antiviral therapy; EVL, everolimus; CSA, cyclosporine [Color figure can be viewed at wileyonlinelibrary.com]
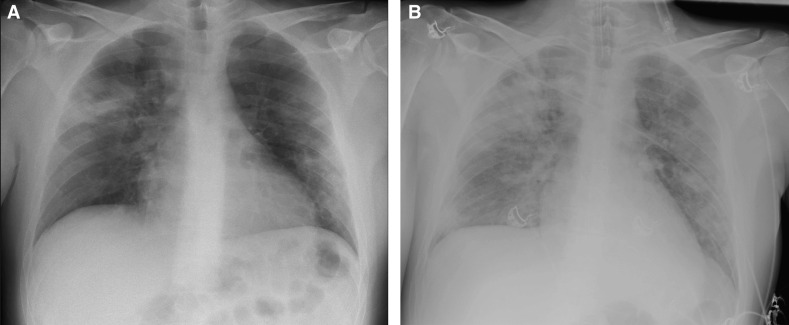
On February 29, 2020, the patient developed a fever, cough, malaise, muscle pain, and headache. He visited his general practitioner and received acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), and tramadol. His complaints progressed over the following days necessitating a visit to the emergency department of Amphia Hospital, a large community hospital, on March 10, 2020. He then complained of dyspnea, with a tachypnea of 30/min, productive cough with sputum without hemoptysis, rhinorrhea, head and muscle ache, nausea, vomiting, and loose stools without abdominal pain. He remained hemodynamically stable. Laboratory examination showed a lymphopenia, acute on chronic kidney injury, elevated inflammatory markers, rhabdomyolysis, elevated transaminases, an acute respiratory alkalosis with hypoxemia, and a high anion gap metabolic acidosis due to renal insufficiency. Chest radiography showed peripheral consolidations in multiple lobes in the lung, highly suspicious for COVID-19 pneumonia ( Figure 2A). Nasopharyngeal swab for SARS-CoV-2 was positive, as was a urine pneumococcal antigen test. Details are summarized in Table 1.
FIGURE 2.
A, Sitting anteroposterior chest radiograph on March 10, 2020, showing peripheral localized consolidations in multiple lobes in the lung suspicious for a viral pathogen. B, Supine anteroposterior chest radiograph on March 14, 2020, showing progressive, peripheral, and bilateral consolidations, primarily in the right upper lobe
TABLE 1.
Patient characteristics upon presentation
| Value | Unit | ||
|---|---|---|---|
| Upper and lower respiratory tract | Tachypnea 30/min with 94% oxygen saturation | ||
| Productive cough with sputum without hemoptysis | |||
| Runny nose and nose congestion | |||
| Headache | |||
| Pulmonary rales over all lung fields | |||
| Gastrointestinal tract | Nausea | ||
| Vomiting | |||
| Loose stools without abdominal pain | |||
| Musculoskeletal tract | Muscle ache | ||
| Complete blood count | Normal leukocyte count | 5.3 | ×109/L |
| Lymphopenia | 0.29 | ×109/L | |
| Acute kidney injury | Creatinine | 3.6 | mg/dL |
| Elevated creatinine kinase | 45 247 | U/L | |
| Elevated lactate dehydrogenase | 901 | U/L | |
| Elevated inflammation markers | Erythrocyte sedimentation rate (ESR) | 65 | mm/h |
| C-reactive protein (CRP) | 143 | mg/L | |
| Elevated liver enzymes | Aspartate aminotransferase (AST) | 329 | U/L |
| Alanine aminotransferase (ALT) | 83 | U/L | |
| Normal bilirubin and cholestatic liver enzyme levels | |||
| Arterial blood gas analysis | Mixed respiratory alkalosis with hypoxemia | ||
| High anion gap metabolic acidosis due to renal insufficiency | |||
| Chest radiograph | Peripherally localized consolidations in multiple lobes in the lung on chest radiograph, suspicious for a viral pathogen | ||
Strict viral isolation followed, oxygen was given by nasal cannula and intravenous cefuroxime was started. Nonetheless, he was persistently dys- and tachypneic, resulting in admittance to the intensive care unit (ICU). He remained respiratory stable with 5 L of oxygen therapy through nasal cannula.
Directly after assessing his QTc time, oral chloroquine (300 mg BID after 600 mg loading dose) and combination antiviral therapy with lopinavir/ritonavir (400/100 mg BID) were initiated, based on RIVM guidelines at the time. Considering interactions with lopinavir/ritonavir, the everolimus dose was reduced from 3 to 2 mg BID. Cefuroxime was replaced by ceftriaxone as part of selective colonic decontamination, which is standard Dutch ICU protocol for intubated patients with expected duration of >24 hours or admission >48 hours. He remained respiratory stable with 5 L/min of oxygen therapy. Discharge to the inpatient clinic followed on March 12, 2020.
Despite everolimus dose reduction at ICU admission, a supratherapeutic trough concentration of 31.1 µg/L was found on March 12, 2020. Subsequently, everolimus was discontinued. His renal function and respiratory status deteriorated on March 14, 2020, necessitating transfer to our transplant center for further treatment. Upon arrival, he appeared very dyspneic with a tachypnea of 38/min and oxygen saturation of 99% with 2 L/min oxygen therapy. Everolimus was persistently elevated (14.4 µg/L) despite cessation of the drug 2 days earlier. Further deterioration with respiratory insufficiency resulted in admittance to the ICU and rapid intubation. A positive end expiratory pressure (PEEP) of 10 cm H2O was needed in combination with a fraction inspired oxygen (FiO2) of 60%. A repeated thoracic radiograph showed progressive bilateral consolidations, most prominent in the right upper lobe (Figure 2B). Differential diagnoses of the novel respiratory deterioration included pulmonary interstitial diseases associated with everolimus toxicity, pneumonitis, and bacterial or opportunistic superinfection acquired in-hospital in combination with acute pulmonary edema. Lopinavir/ritonavir and chloroquine, which he had for 4 days, were ceased; antibiotics were continued; and loop diuretics were initiated. A pulmonary computed tomography (CT) scan and bronchoscopic lavage were considered but halted in anticipation of due course.
Four days later, he was successfully extubated with adequate oxygenation with 4 L/min oxygen therapy and discharged to the COVID-19 ward. Everolimus concentrations decreased gradually and were below the detection limit (<0.5 µg/L) from March 23, 2020 on. The everolimus concentrations during admission are displayed in Figure 1B. Cyclosporine-based immunosuppression was introduced on March 23, 2020. Thereafter, he recovered steadily. However, his renal function has not yet returned to baseline, probably due to a combination of NSAID use, everolimus toxicity, rhabdomyolysis, and/or viral tubulo-interstitial nephritis. Twenty-four-hour urinalysis showed 630 mg proteinuria without leukocyturia or erythrocyturia. On March 24, 2020, 15 days after first admission, he could be discharged with self-quarantine precautions and follow-up at our outpatient clinic.
3. DISCUSSION
It is currently unknown whether transplant recipients with COVID-19 have different symptoms as compared to immunocompetent people.8 A recent report stated an onset of abdominal pain prior to development of respiratory complaints.9 Our case, however, had similar complaints as previous immunocompetent cases.10 , 11
For renal transplant recipients with COVID-19, there are various hypotheses regarding therapeutic targets. First, no association between angiotensin-converting enzyme (ACE)-2-receptor upregulation and use of ACE inhibitors, angiotensin receptor blockers (ARBs), or NSAIDs has been found in humans. There is no evidence that ACE inhibitors and ARBs worsen COVID-19. Contrarily, withdrawal of renin-angiotensin-aldosterone-system (RAAS) inhibitors may be harmful in high-risk patients.12 Second, the potential withdrawal or reduction of immunosuppression to facilitate SARS-CoV-2 clearance seems important. The best strategy, however, remains unclear. Our patient experienced extensive everolimus exposure, in contrast to a previous case where triple maintenance immunosuppressive therapy was replaced with pulsatile intravenous methylprednisolone.8 Recent reports from China also showed lower mortality in COVID-19-patients receiving steroids for ARDS. Contrarily, previous studies on MERS showed longer viral presence in patients treated with high-dose corticosteroids. Therefore, we think a (stepwise) reduction in immunosuppression may be best to prevent prolonged viral presence and spreading, carefully balancing risk of immune reconstitution and rejection with development of ARDS. For immunosuppression titration, we favored calcineurin inhibitor (CNI)-based immunosuppression with prednisolone. We preferred cyclosporine given the acute kidney injury with mild proteinuria, absence of pulmonary adverse effects, and its potential in vitro antiviral effect against coronaviruses, although it is unclear whether in vivo tissue concentrations are sufficient for any clinical efficacy. If diarrhea is present, intravenous CNIs might be preferred.
mTORis may lower mucosal immunity more specifically as evidenced by adverse events such as mucosal ulcers and interstitial lung disease. Moreover, COVID patients often show signs of renal involvement and proteinuria,13 which we deemed a contraindication for everolimus continuation. Also, considering mycophenolate’s adverse mucosal effects of hypogammaglobulinemia, bronchiectasis, diarrhea, diminished viral gastrointestinal infection clearance, and even incidental colitis, in combination with often observed leukopenia, we decided against its use in this case.
Third, evidence-based antiviral treatment for COVID-19 is lacking. Whereas experimental pharmacological therapy with a limited scientific basis may be potentially beneficial, risk-benefit assessments remain pivotal for complex solid organ transplant recipients. As illustrated in this case, considerations should include drug interactions with immunosuppressive therapy. At admission of our patient, chloroquine and lopinavir/ritonavir were initiated according to Dutch RIVM guidelines at that time. In our opinion, lopinavir/ritonavir is better avoided, as the SARS-CoV-2 protease differs from that of human immunodeficiency virus (HIV), questioning the mechanistic rationale for such experimental treatment, and because of dangerous in vivo interactions and recent evidence of inefficacy.14 We do, however, consider (hydroxy)chloroquine for our recipients with hypoxemia and/or high-suspicion radiological features for benefit of doubt and limited interactions.
Regarding potential interactions, CNIs and mTORis are cleared mainly via cytochrome P450 enzyme 3A5 (CYP3A5), CYP3A4, and P-glycoprotein (P-gp). Also, the CYP3A family is involved in prednisolone metabolism.15 Contrarily, mycophenolate metabolism relies on glucuronidation by uridine diphosphate glucuronosyltransferase enzyme 1A9 (UGT1A9) and UGT2B7, whereas contribution of other UGT or CYP3A enzymes is minimal.16
Investigational trials on pharmacological COVID-19 treatment evolve rapidly and include various protease inhibitors (such as lopinavir/ritonavir) or other drugs that inhibit CYP3A and P-gp, which raises new concerns for drug interactions. These may thus dramatically elevate exposure of CNIs or mTORis, as illustrated in our case. For CNIs, drastic dosage reduction of up to 120-fold and prolonged dosing intervals were needed in renal transplant recipients with HIV.17 Microdosing strategies have been suggested for tacrolimus (0.5-1.0 mg once weekly) and cyclosporine (25 mg every 1-2 days).17 Overall, clinical response, drug levels, and signs for drug toxicity should be monitored closely during coadministration of strong CYP3A inhibitors and CNIs or mTORis.17 Accordingly, the US Food and Drug Administration (FDA) labels of tacrolimus, cyclosporine, everolimus, and sirolimus warn for coadministration with strong CYP3A inhibitors and recommend regular TDM. Also, the prednisolone label suggests dose reduction when combined with strong CYP3A inhibitors.
Immediate withdrawal of mTORi therapy and close monitoring of blood concentrations, clinical status, and signs of drug toxicity are recommended when antiviral therapy with strong CYP3A and/or P-gp inhibitory properties is considered. Subsequently, microdosed cyclosporine or tacrolimus can be initiated when subtherapeutic mTORi levels are achieved. However, caution is needed, and cyclosporine should not be initiated too early after everolimus withdrawal, as this may increase everolimus concentrations via CYP/P-gp interaction. For further reference during the COVID-19 pandemic, an overview of drug interactions with investigational COVID-19 therapies is hosted by the University of Liverpool (http://www.covid19-druginteractions.org/).
If chloroquine therapy is considered, up to 3-fold increases in cyclosporine levels can be expected.18 , 19 The mechanism remains unknown albeit confirmed after rechallenge. The FDA chloroquine label warns for increased cyclosporine levels when coadministered, whereas no such effect is seen with tacrolimus. Also, patients with impaired renal function might require reduced-dose chloroquine and maximal therapy of 5 days. We recommend close cyclosporine TDM because chloroquine’s extensive half-life of 1-2 months yields interaction potential far beyond withdrawal. Additionally, chloroquine therapy is associated with mild QTc-interval prolongation,20 possibly augmented if combined with tacrolimus- or propofol-associated QTc-prolongation. Hence, we recommend QTc-interval evaluation when combined with tacrolimus and vigilance for chloroquine accumulation in patients with impaired renal function.
In contrast to typical COVID-19 cases, our patient required mechanical ventilation for only 4 days, which can be explained by various hypotheses. Possibly, severe ARDS was partially prevented by long-term maintenance immunosuppression diminishing pulmonary inflammation. A long-term immunoregulatory effect of the mesenchymal stromal cells 4 years after transplantation was deemed less likely. Alternatively, the respiratory deterioration appeared quick and reversible in close association with everolimus levels. Hence, everolimus-associated pulmonary toxicity cannot be excluded. Unfortunately, his clinical status at that time did not allow a pulmonary CT scan.
We demonstrated important clinical and pharmacokinetic aspects that play a role in renal transplant recipients with COVID-19. This awareness can assist physicians in their decision-making during the current pandemic. However, more experience is required to enable evidence-based, efficacious, and safe COVID-19 care in renal transplant recipients.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Footnotes
Soufian Meziyerh and Tom C. Zwart contributed equally to this work.
REFERENCES
- 1.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 2.Al Ghamdi M, et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic [published online ahead of print 2020]. Liver Transpl. 2020. 10.1002/lt.25756. [DOI] [PubMed]
- 4.de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Medicine. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollaard E, de Boer G, van Dissel S. Drug treatment options for hospitalized patients with COVID-19. https://web.archive.org/web/20200310084722/https://lci.rivm.nl/covid-19/bijlage/medicamenteuze-behandelopties. Published 2020. Accessed March 10, 2020.
- 8.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [published online ahead of print 2020]. Am J Transplant. 2020. 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed]
- 9.Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed]
- 10.Xu T, Chen C, Zhu Z, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naicker S, Yang C-W, Hwang S-J, et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed]
- 15.Bergmann TK, Barraclough KA, Lee KJ, et al. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation. Clin Pharmacokinet. 2012;51(11):711–741. doi: 10.1007/s40262-012-0007-8. [DOI] [PubMed] [Google Scholar]
- 16.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46(1):13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 17.van Maarseveen EM, Rogers CC, Trofe-Clark J, et al. Drug-drug interactions between antiretroviral and immunosuppressive agents in HIV-infected patients after solid organ transplantation: a review. AIDS Patient Care STDS. 2012;26(10):568–581. doi: 10.1089/apc.2012.0169. [DOI] [PubMed] [Google Scholar]
- 18.Nampoory N, Nessim J, Gupta RK, et al. Drug interaction of chloroquine with ciclosporin. Nephron. 1992;62(1):108–109. doi: 10.1159/000187007. [DOI] [PubMed] [Google Scholar]
- 19.Finielz P, Gendoo Z, Chuet C, et al. Interaction between cyclosporin and chloroquine. Nephron. 1993;65(2):333. doi: 10.1159/000187506. [DOI] [PubMed] [Google Scholar]
- 20.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.