Abstract
The number of patients requiring tracheal intubation rose dramatically in March and April 2020 with the COVID‐19 outbreak. Our thoracic surgery department has seen an increased incidence of severe pneumomediastinum referred for surgical opinion in intubated patients with COVID‐19 pneumonitis. Here we present a series of five patients with severe pneumomediastinum requiring decompression therapy over a 7‐day period in the current COVID‐19 outbreak. We hypothesise that the mechanism for this is the aggressive disease pathophysiology with an increased risk of alveolar damage and tracheobronchial injury, along with the use of larger‐bore tracheal tubes and higher ventilation pressures. We present this case series in order to highlight the increased risk of this potentially life‐threatening complication among the COVID‐19 patient cohort and offer guidance for its management to critical care physicians.
Keywords: acute respiratory distress syndrome, COVID‐19, pneumomediastinum, surgical emphysema, tracheal injury
Introduction
The number of patients requiring tracheal intubation or tracheostomy has risen dramatically over March and April 2020 with the COVID‐19 outbreak. (ICNARC data, https://www.icnarc.org/Our‐Audit/Audits/Cmp/Reports, accessed 30/4/20). Pneumomediastinum is a rare, potentially life‐threatening condition defined as the presence of air within the mediastinum. It is commonly associated with barotrauma or trauma to the oesophageal, tracheobronchial, lung or pleural space and is associated with a poor prognosis. Over the past month, we have seen an increase in the incidence of severe pneumomediastinum and surgical emphysema following tracheal intubation and ventilation in patients with confirmed COVID‐19 referred to our tertiary thoracic unit. This is a novel finding with few published data; however, it is one which carries a high morbidity and mortality. We present a prospective case series to describe the presentation, management and outcome of these cases.
Case Series
We present the cases of five patients, with proven COVID‐19, who developed severe pneumomediastinum after intubation and were referred to our tertiary thoracic surgery department over a 7 day period from 1 to 7 April 2020. All of the information presented was provided as part of the referral process; follow‐up information was obtained by contacting the parent intensive care team. We recorded baseline patient characteristics including: comorbidities; ventilation data; information regarding the signs of pneumomediastinum and its diagnosis; ongoing management; and outcome. Consent for the use of anonymised data and imaging was obtained from the next of kin.
Five patients were included in this case series (Table 1). Patients were male and median (range) age was 60 years 60 (38‐70) years. All were invasively ventilated for respiratory compromise with severe hypoxaemia, despite maximal ward‐based oxygen therapy (Pa02 on arterial sample < 6kPa) after exhibiting typical symptoms of COVID‐19 pneumonitis. Two patients were admitted to the intensive care unit (ICU) from our Emergency Department, two were transferred (already ventilated) from a local district general hospital and one was a remote consultation in a distant ICU which did not have a local thoracic surgery service. No patients had a known history of lung disease. The Charlson comorbidity score of the patients ranged from 0 to 2 and performance status rated 0 in all cases.
Table 1.
Case series
| Patient | Age; y | Sex | Comorbidities | Admission reason | Day intubated (since admission) | Internal diameter of TT (mm) | Ventilation parameters [PEEP, peak pressure] (cmH2O) | Signs of pneumomediastinum (days since intubation) | Time of diagnosis from intubation (modality) | Severity | Bronchoscopy evidence of tracheal injury? | Management (days since intubation) | Days ventilated | Outcome (days since intubation) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Maximum | ||||||||||||||
| 1 | 70 | M | Hypothyroidism, smoker (50 pack years) | 7 days SOB, pyrexia, cough | D1 | 8 | 12, 22 | 14, 28 | Surgical emphysema, increasing FiO2 requirements and unstable BP – likely tamponade (D14) | 14 days (CTPA) | Severe | No injury seen | Bilateral intrapleural chest drains and bilateral subcutaneous drains (D14) | 30 |
Weaning tracheostomy Drains removed |
| 2 | 60 | M | IDDM, pancreatitis | Admission with HHS, 7 days SOB, pyrexia | D2 | 9 | 8, 27 | 12, 32 | Surgical emphysema (D2), increasing FiO2 requirements and unstable BP ‐ likely tamponade (D4) | 4 h (CTPA) | Severe | Right main bronchus injury | Bilateral intrapleural chest drains and bilateral subcutaneous drains (D4) | 16 |
Weaning tracheostomy Drains removed |
| 3 | 38 | M | Nil | 14 days SOB, pyrexia, cough | D3 | 9 | 10, 20 | 10, 31 | Surgical emphysema (D2) |
2 days (CXR) 5 days (CTPA) |
Severe | Not performed |
ECMO (D4) Bilateral chest drains (D19) |
20 | Patient died (D20) |
| 4 | 51 | M | Asthma | 3 days SOB, cough | D1 | 9 | 12, 30 | 12, 34 |
Left sided pneumothorax (D2) increasing FiO2 requirements and unstable BP after proning |
2 days (CXR) | Moderate to Severe | Not performed | Left intrapleural chest drain (D3) | 4 | Patient died (D4) |
| 5 | 60 | M | Surveillance for early prostate cancer | 2 days SOB | D1 | 8–9 | 8, 16 | 12, 26 | Surgical emphysema (D6) | 6 days (CXR + CTPA) | Moderate to severe | No injury seen | Conservative | 25 |
Pneumomediastinum and surgical emphysema resolved on repeat CTPA Stepped down to ward |
TT, tracheal tube; PEEP, positive end‐expiratory pressure; SOB, shortness of breath; IDDM, insulin dependent diabetes mellitus; HHS, hyperosmolar hyperglycaemic state; FiO2, fraction of inspired oxygen; BP, blood pressure; CTPA, computed tomography pulmonary angiogram; CXR, chest X‐ray; ECMO, extra corporeal membranous oxygenation.
All patients were invasively ventilated within 3 days of admission by the ICU team. The trachea was intubated using direct laryngoscopy in four patients, the videolaryngoscope was used in one patients; no bougies or stylets were used. In three patients, a size‐9 tracheal tube was used at the time of initial intubation. In one patients, a size‐8 was used initially, this was exchanged for a size‐9 (over a bougie) after 2 days of mechanical ventilation for better oxygen delivery. In all patients, assist pressure control ventilation was utilised with Drager V500 ventilators (Drager, Lubeck, Germany). Initial positive end‐expiratory pressure (PEEP) ranged from 8 to 12 cmH2O, with mean airway pressures of 13–15 cmH2O and peak airway pressures of 22–30 cmH2O. The highest ventilatory pressures were a PEEP of 10–14 cmH2O, mean airway pressures of 22–31 cmH2O and peak airway pressures of 28–34 cmH2O.
Time to develop pneumomediastinum following tracheal intubation ranged from 4 h to 14 days. Four out of the five patients presented with obvious subcutaneous emphysema and increasing oxygen requirements. Pneumomediastinum was diagnosed due to progressive surgical emphysema and confirmed with chest X‐ray (Fig. 1) or computed tomography (CT) imaging (Fig. 2). One patient had a tracheal injury visible on CT scan which was verified via flexible bronchoscopy. Thoracic surgical input was sought due to concerns over extubation, tamponade and potential tension pneumothorax. Bilateral intra‐pleural and subcutaneous chest drains were placed in four patients to treat the sequelae of presumed tracheal injury which was otherwise managed conservatively. All chest drains were placed on suction and the use of bilateral ‘blow holes’ attached to vacuum assisted closure pumps were advised to ensure a closed circuit in order to avoid aerosolising the virus as best possible [1].
Figure 1.
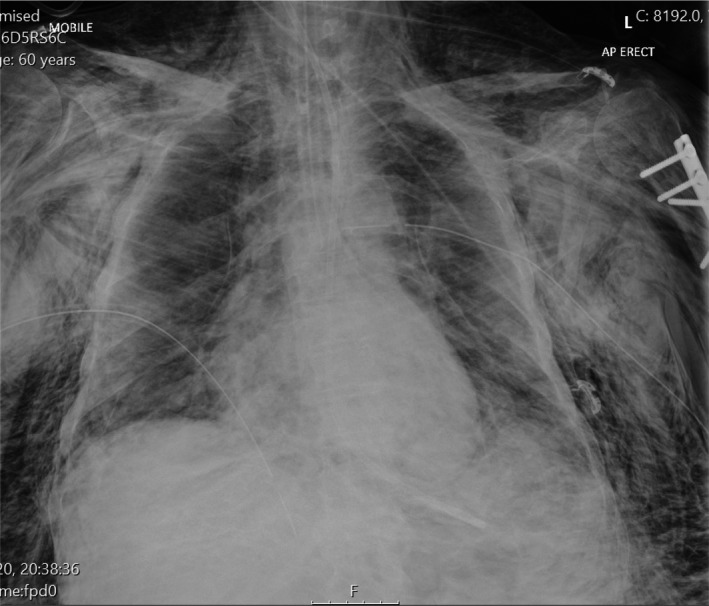
Chest X‐ray demonstrating significant surgical emphysema, pneumomediastinum and pneumopericardium in an intubated COVID‐19 patient. Bilateral intra‐pleural chest drains can be visualised.
Figure 2.
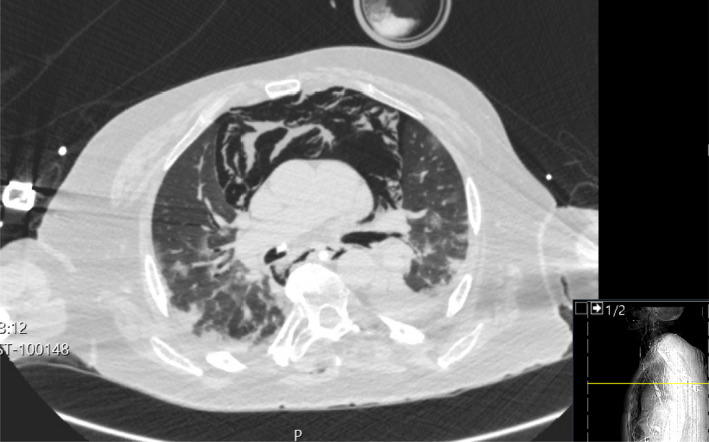
Axial section of computed tomography scan of COVID‐19 thorax demonstrating severe pneumomediastinum. There is mediastinal shift and a reduction in lung volume with a flattening of the normal curvature of the right heart border suggestive of tamponade. Note the bilateral infiltrates typical of COVID‐19 pneumonitis.
At the time of writing, two out of five patients had passed away. One patient died after being put on extra corporeal membrane oxygenation (ECMO) with a rising lactate and right heart failure thought secondary to pulmonary hypertension. The second patient had multi‐organ failure and was not a candidate for ECMO. Three patients were successfully decannulated with removal of chest drains and are currently clinically stable.
Discussion
Pneumomediastinum is usually a benign and self‐limiting condition and occurs when extra‐luminal gas enters the mediastinum [1, 2]. In high‐risk COVID‐19 patients, many of whose lungs are mechanically ventilated with high positive pressures, there is a risk of severe mediastinal emphysema, pneumothorax and pneumopericardium which can mimic cardiac tamponade. Emphysema usually tracks superiorly and in extremely severe cases may constrict the main airway and impede blood flow in head and neck vessels [1].
The causes of pneumomediastinum are varied. It is most often caused by increased airway pressures, secondary to mechanical ventilation or airway obstruction; however, other causes include: a rise in intra‐thoracic pressure (such as from the Valsalva manoeuvre); strenuous activity; severe vomiting (diabetic keto‐acidosis, anorexia nervosa); trauma to the thoracic cavity; oesophageal rupture; thoracic and head and neck surgery, particularly with resultant tracheobronchial injury; and alveolar injury due to underlying disease such as infection and sarcoidosis [3]. During the initial period of COVID‐19 infections, we have seen a number of patients with severe pneumomediastinum.
There are currently three published case reports of spontaneous pneumomediastinum in the setting of COVID‐19 [2, 4, 5]. In the first case report of a 38‐year‐old male, pneumomediastinum developed on day 11, this was noted to herald worsening disease, however the pneumomediastinum resolved with conservative management [2]. The second case report describes the case of a 38‐year‐old male in Wuhan who was found to have multiple bullae, including a giant bulla, causing surgical emphysema and pneumomediastinum. This was thought secondary to raised intra‐thoracic pressures after coughing and was also managed conservatively [4]. The third case report details a female patient in whom a CT scan showed pneumomediastinum. This was obtained 12 days after symptom onset and there was no intervention for the pneumomediastinum. The patient subsequently died two days later of respiratory failure [5].
A review of chest X‐rays in COVID‐19 patients has suggested there may be multiple cases of pneumomediastinum, with alveolar rupture secondary to positive pressure ventilation as a possible cause [6]. However, CT scan remains the definitive diagnostic tool. This will demonstrate subcutaneous emphysema, pneumopericardium and potential tracheobronchial injuries alongside the bilateral infiltrates typical of COVID‐19 [7] (Fig. 2) Standard chest X‐rays will reveal subcutaneous emphysema (Fig. 1), as well as rim of air which is often prominent on the left border of the heart suggesting a pneumopericardium. Naclerio’s V sign describes a similar outline along the descending aorta and the left hemi‐diaphragm [8]. Although chest X‐ray is the diagnostic standard for pneumomediastinum, half of all cases may be missed without a lateral film [9], which was not feasible in the ventilated ICU patients described in this series. A lateral chest X‐ray would identify air anterior to the mediastinum (pneumo‐precardium). The ‘ring‐around‐the‐artery’ sign (lucency around the right pulmonary artery) might also be indicative of pneumomediastinum in a lateral film. [8]. Computed tomography imaging clearly delineates the extent of surgical emphysema (Fig. 2), and has been shown to be more reliable than chest X‐ray for this purpose [10]. Figure 2 clearly demonstrates severe mediastinal emphysema and the associated dorsal mediastinal shift.
The pathophysiology of COVID‐19 is purported to involve a cytokine storm in the airway that cause destruction of the alveoli. This may lead to spontaneous pneumomediastinum through Macklin’s phenomenon. Macklin described how alveolar air which is released from alveolar rupture tracks along peri‐bronchial vascular sheaths towards the mediastinum [11]. COVID‐19 patients who are mechanically ventilated via a tracehal tube for respiratory failure are likely to be predisposed to developing pneumomediastinum as a result of the natural history of the disease. Furthermore, the high PEEP values seen in this case series may correlate to an increased risk of barotrauma, as is widely reported in the literature [12].
The management of pneumomediastinum was carried out alongside treatment of ongoing COVID‐19 infection. One may adopt a conservative approach as described in the previous case reports above. Other options include: needle aspiration; chest drain insertion; fenestrated subcutaneous catheters; or vacuum assisted closure dressings to the supra‐clavicular space [1]. Due to the extension of air caudally, the most favourable location for decompression is superiorly, adjacent to the thoracic inlet [1]. Continued clinical and radiological assessment needs to inform any interventions in this patient group. In severe and worsening pneumomediastinum, with associated mediastinal shift and respiratory compromise, we would recommend the insertion of subcutaneous drains and bilateral intra‐pleural chest drains to prevent life threatening complications. This was carried out in three of the patients in this series. In the context of COVID‐19, a closed chest drain system should be used where possible to prevent aerosolising of the virus. This may be achieved by applying wall suction to the underwater seal. If life threatening tamponade does occur, then immediate needle decompression is required.
The presumed aetiology in these patients was two‐fold: alveolar damage and tracking of air (through the previously described Macklin’s phenomenon as well as iatrogenic tracheal injury following tracheal intubation), which was visualised radiologically and bronchoscopically in one patient in this series. It is hypothesised that sizing up of the tracheal tube may be traumatic to the membranous wall of the trachea which is weakened in COVID‐19 cases (four of our patients had a size‐9 tracheal tube).
COVID‐19 is recognised as causing central and upper airway inflammation and oedema leaving patients potentially vulnerable to injury from instrumentation. Furthermore, expeditious intubation due to severe hypoxaemia in emergent settings could be a contributory factor to tracheobronchial injury. Where major proximal tracheal injury is suspected, it is worth considering advancing the tracheal tube distal to the injury. This is best achieved by bronchoscopic control, recognising that bronchoscopy places the operator at risk of major aerosolised viral exposure.
Repositioning manoeuvres may also contribute to tracheobronchial injury. Patient 4 in this series developed pneumomediastinum immediately following prone positioning manoeuvres. The risks of proning mechanically ventilated patients for optimising ventilation perfusion mismatch are well documented [13] and it is possible that the increased number of patients requiring this manoeuvre has led to an increased incidence of tracheobronchial injury.
Pneumomediastinum is a rare but well‐described phenomenon in acute respiratory distress syndrome (ARDS). A retrospective review of 1781 CT scans of patients invasively ventilated for ARDS found that 7.4% had radiologically‐evident pneumomediastinum [14]. The severity of these cases is not reported. One of the striking features of this case series is the clinical severity of the pneumomediastinum and surgical emphysema in the COVID‐19 cohort as depicted. In a prospective follow‐up of 75 patients affected with severe acute respiratory distress syndrome (SARS) in the 2003 outbreak (secondary to a strain of coronavirus), it was noted that 12% of patients had developed a spontaneous pneumomediastinum. Of note, in this study, no relationship was identified between the incidence of pneumomediastinum, and intubation or the extent of PEEP used. In theory, the pulmonary lesions abutting the mediastinum or located peripherally may rupture to cause pneumomediastinum [15]. Recent CT analysis indicated that lung lesions in COVID‐19 patients are predominantly peripheral (reported as 93.6% of a 53 patient cohort), although no pneumomediastinum was identified in this group [16].
Pneumomediastinum has been demonstrated to be the initial manifestation of barotrauma in intubated patients, and a predictor for the development of pneumothorax. Studies into pulmonary barotrauma have suggested that pneumomediastinum is a negative prognostic indicator in intubated patients [17]. Barotrauma in all its manifestations has been proven to increase mortality in patients with ARDS [18].
Conclusion
Although rare, development of pneumomediastinum in COVID‐19 patients may be a negative prognostic marker. The combination of alveolar damage and weakness of the membranous wall of the trachea, intubation potentially in emergent scenarios, frequent proning and barotrauma from high ventilator pressures, may predispose this patient cohort to severe pneumomediastinum. Our team has worked hand in hand with the intensive care team to carefully monitor the patients affected and their ongoing management. Based on our recent experience, we would advocate the use of bilateral chest and subcutaneous drains in the management of severe pneumomediastinum for decompression.
Acknowledgements
No competing interests declared.
References
- 1. Byun CS, Choi JH, Hwang JJ, Kim DH, Cho HM, Seok JP. Vacuum‐assisted closure therapy as an alternative treatment of subcutaneous emphysema. Korean Journal of Thoracic and Cardiovascular Surgery 2013; 46: 383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou C, Gao C, Xie Y, Xu M. COVID‐19 with spontaneous pneumomediastinum. Lancet Infectious Diseases 2020; 20: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bejvan SM, Godwin JD. Pneumomediastinum: old signs and new signs. American Journal of Roentgenology 1996; 166: 1041–8. [DOI] [PubMed] [Google Scholar]
- 4. Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID‐19 pneumonia. Korean Journal of Radiology 2020; 21: 541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Su X, Zhang T, Zheng C. Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID‐19) pneumonia. Korean Journal of Radiology 2020; 21: 627–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X‐ray in coronavirus disease‐19 (COVID‐19): A pictorial review. Clinical Imaging 2020; 64: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology 2020; 295: 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zylak CM, Standen JR, Barnes GR, Zylak CJ. Pneumomediastinum revisited. Radiographics 2000; 20: 1043–105. [DOI] [PubMed] [Google Scholar]
- 9. Koullias GJ, Korkolis DP, Wang XJ, Hammond GL. Current assessment and management of spontaneous pneumomediastinum experience in 24 adult patients. European Journal of Cardio‐Thoracic Surgery 2004; 25: 852–5. [DOI] [PubMed] [Google Scholar]
- 10. Okada M, Adachi H, Shibuya Y, Ishikawa S, Hamabe Y. Diagnosis and treatment of patients with spontaneous pneumomediastinum. Respiratory Investigation 2014; 52: 36–40. [DOI] [PubMed] [Google Scholar]
- 11. Wintermark M, Schnyder P. The Macklin effect: a frequent etiology for pneumomediastinum in severe blunt chest trauma. Chest Journal 2001; 120: 543–7. [DOI] [PubMed] [Google Scholar]
- 12. Eisner MD, Taylor Thompson B, Schoenfeld D, Anzueto A, Matthay MA. Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2002; 165: 978–82. [DOI] [PubMed] [Google Scholar]
- 13. Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest 2017; 151: 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simon M, Braune S, Laqmani A, et al. Value of computed tomography of the chest in subjects with ARDS: A retrospective observational study. Respiratory Care 2016; 61: 316–23. [DOI] [PubMed] [Google Scholar]
- 15. Peiris JSM, Chu CM, Cheng VCC, et al. Clinical profession and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 2003; 361: 1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan CS, Zi B, Shuo Y, et al. (COVID‐19): evaluation on thin‐section CT. Academic Radiology 2019; 2020: 609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation; patterns and risk factors. Chest Journal 1992; 102: 568–72. [DOI] [PubMed] [Google Scholar]
- 18. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000; 342: 1301–8. [DOI] [PubMed] [Google Scholar]


