Abstract
As of April 20, 2020, over time, the COVID‐19 pandemic has resulted in 157 970 deaths out of 2 319 066 confirmed cases, at a Case Fatality Rate of ~6.8%. With the pandemic rapidly spreading, and health delivery systems being overwhelmed, it is imperative that safe and effective pharmacotherapeutic strategies are rapidly explored to improve survival. In this paper, we use established and emerging evidence to propose a testable hypothesis that, a vicious positive feedback loop of des‐Arg(9)‐bradykinin‐ and bradykinin‐mediated inflammation → injury → inflammation, likely precipitates life threatening respiratory complications in COVID‐19. Through our hypothesis, we make the prediction that the FDA‐approved molecule, icatibant, might be able to interrupt this feedback loop and, thereby, improve the clinical outcomes. This hypothesis could lead to basic, translational, and clinical studies aimed at reducing COVID‐19 morbidity and mortality.
Keywords: bradykinin, bradykinin receptor, coronavirus, icatibant, inflammation, injury
Abbreviations
- ACE
angiotensin converting enzyme
- APP
aminopeptidase‐P
- B1R
bradykinin‐B1‐receptor
- B2R
bradykinin‐B2‐receptor
- BK
bradykinin
- CoV
coronavirus
- COVID‐19
coronavirus disease 19
- DABK
des‐Arg(9)‐bradykinin
- DPP4
dipeptidyl peptidase‐4
- ER
endoplasmic reticulum
- FDA
United States Food and Drug Administration
- HAE
hereditary angioedema
- IL
interleukin
- SARS
severe acute respiratory syndrome
According to data reported by the World Health Organization through its COVID‐19 homepage, as of April 20, 2020, 2:00 am CEST, out of 2 319 066 confirmed cases over time, there have been 157 970 deaths, putting the Case Fatality Rate at ~6.8%. 1 As the COVID‐19 pandemic is rapidly spreading, and health delivery systems are being overwhelmed by the large numbers of patients needing acute care for breathing difficulty, it is imperative that safe and effective pharmacotherapeutic strategies are rapidly explored to improve survival. 2 , 3 Since time is of the essence to reduce mortality in patients with COVID‐19 respiratory complications, repurposing FDA‐approved drugs that have a good safety profile for off‐label and/or compassionate use should be a strategic priority. 4
It is in this context that we propose a testable hypothesis for dysregulated bradykinin (BK) signaling in COVID‐19 respiratory complications. Through our hypothesis, we hope that researchers and clinicians would be able to identify candidate drugs for off‐label and/or compassionate use in patients with unremitting respiratory distress from COVID‐19.
Based on our examination of basic and clinical studies, we hypothesize that dysregulated BK signaling is involved in COVID‐19 respiratory complications for the following reasons (also see Figure 1):
The severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), which causes COVID‐19, is known to enter host cells in the respiratory system via the transmembrane protein, angiotensin converting enzyme 2 (ACE2) 5 , 6
SARS‐CoV infection depletes ACE2 7
ACE2 depletion increases levels of des‐Arg(9)‐bradykinin (DABK), which is a bioactive metabolite of BK that is associated with lung injury and inflammation 8 , 9 , 10
A possible role for BK in COVID‐19 respiratory distress is consistent with established evidence that, BK, histamine, and serotonin, have for long been known as key mediators of acute lung inflammation and respiratory distress 11
FIGURE 1.
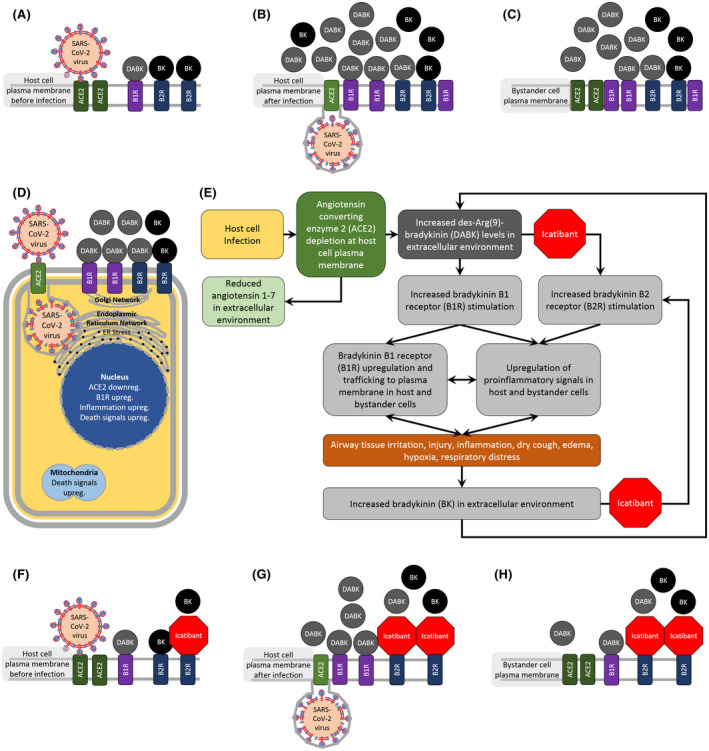
Hypothesized role for dysregulated bradykinin signaling in COVID‐19 respiratory complications and the potential benefit of bradykinin receptor blockers. SARS coronavirus‐2 (SARS‐CoV‐2), the virus that causes coronavirus disease 19 (COVID‐19), is known to enter host cells in the respiratory system via the transmembrane protein, angiotensin converting enzyme 2 (ACE2) 5 , 6 (Panel A). SARS‐CoV infection depletes ACE2 at the plasma membrane of infected cells 7 (Panel B). In the extracellular environment of both infected cells as well as neighboring bystander cells, ACE2 depletion increases the levels of des‐Arg(9)‐bradykinin (DABK), which is a bioactive metabolite of bradykinin (BK) that is associated with airway inflammation 8 (Panels B, C). SARS‐CoV infection severely affects host cell homeostasis, 53 by triggering endoplasmic reticulum stress, 54 mitochondrial death signaling, 55 downregulation of ACE2, 7 upregulation of pro‐inflammatory genes, 56 and nuclear death signals, 57 which ultimately lead to cell death 53 (Panels D, E). Cellular injury and inflammation induces BK‐B1‐receptor (B1R) upregulation and trafficking to the plasma membrane, which amplifies DABK‐mediated inflammation and injury 58 , 59 (Panel D). Tissue injury and inflammation also increases BK levels and BK‐B2‐receptor (B2R) stimulation 59 , 60 (Panels D, E). Our testable hypothesis for dysregulated BK signaling in COVID‐19 respiratory complications is that, ACE2 depletion in SARS‐CoV‐2‐infected cells causes DABK accumulation in the extracellular environment of infected and neighboring bystander cells, which triggers a vicious positive feedback loop of inflammation and injury leading to even greater levels of DABK‐ and BK‐mediated inflammation and injury (Panel E). DABK not only binds strongly to B1Rs, through which it exerts downstream effects, but also binds weakly to B2Rs in certain tissues, and exerts effects that are blocked by the B2R blocker, icatibant 12 , 13 (Panel E). Since there are currently no FDA‐approved drugs that selectively block DABK signaling through B1Rs, we provide a testable prediction that, off‐label use of FDA‐approved icatibant, will at least partially interrupt the positive feedback loop of DABK‐ and BK‐mediated inflammation → injury→inflammation, and improve clinical outcomes in patients with COVID‐19 respiratory complications (Panels E‐H). Bidirectional arrows suggest that, these processes are likely to aggravate each other and be part of smaller positive feedback loops
Experimental evidence suggests that, most downstream effects of DABK are mediated through its binding to the BK‐B1‐receptor (B1R). However, DABK not only binds strongly to B1Rs, but also binds weakly to the BK‐B2‐receptor (B2R) in certain tissues, and exerts downstream effects that are blocked by the B2R blocker, icatibant. 12 , 13
Therefore, the off‐label use of the B2R blocker, icatibant, seems promising for patients with unremitting respiratory distress caused by COVID‐19. Icatibant (Trade Name: FIRAZYR; Takeda, Tokyo, Japan) is a drug that has been approved by the United States Food and Drug Administration (FDA) and other regulatory bodies, for the treatment of angioedema episodes in patients (18 years and older) with hereditary angioedema (HAE). 14 Icatibant is thought to work by binding to B2Rs and blocking the downstream activity of BK in a variety of cells, including those present in blood vessels and the airway. 15 Icatibant is effective in treating breathing difficulty in patients presenting with angioedema, including angioedema caused by angiotensin converting enzyme (ACE) inhibitors taken for hypertension. 16 It might be purely coincidental that COVID‐19 causes a “dry cough” 17 ––a rare but characteristic side effect of ACE inhibitors, which is linked to BK. 18
Icatibant has been shown to be safe and effective, with side effects and adverse reactions being rare when used in the context of angioedema. 19 A human study on the off‐label use of icatibant to treat allergic rhinitis showed that, the drug significantly reduced grass pollen antigen‐induced hyperresponsiveness to histamine, which was linked to icatibant inhibiting interleukin‐8 (IL‐8) release. 20 The fact that IL‐8 is implicated in acute lung injury and respiratory distress, further supports the empirical use of icatibant in the treatment of unremitting respiratory distress in COVID‐19. 21
From a scientific standpoint, analyzing plasma levels of BK and DABK in patients with respiratory complications from COVID‐19, might help support our hypothesis. 22 , 23 It might also be useful to retroactively obtain data on patients who have been treated recently with icatibant for angioedema, while having COVID‐19 as a comorbidity, to ascertain whether or not COVID‐19 respiratory symptoms decreased after icatibant administration. In addition, it would be worth closely monitoring outcomes in patients with COVID‐19 who take ACE inhibitors (for hypertension), dipeptidyl peptidase‐4 (DPP4) inhibitors (for diabetes mellitus), or neprilysin inhibitors (for heart failure), since these drugs are known to interfere with BK breakdown and thus increase the BK bioavailability. 24
It is possible that molecules other than icatibant, which act on BK signaling pathways, might also be able to reduce the respiratory distress in COVID‐19. For example, blocking DABK's main target, B1R, might produce better outcomes. 25 However, at this time, B1R blockers (eg, orally‐active BI‐113823) have only been tested in animals and in limited human trials. 26 , 27 , 28 Nonetheless, B2R blockade has been shown to be effective in the context of airway hyper responsiveness and respiratory distress in animal models; and, DABK has been shown to act on the B2R in some tissues. 12 , 13 , 25 Inhibiting BK production with the FDA‐approved drug, ecallantide, also seems promising, although it carries a risk of anaphylaxis in some patients. 29 Increasing plasma levels of aminopeptidase‐P (APP), an enzyme that degrades BK and DABK, could also be tested as a benign intervention aimed at accelerating BK and DABK degradation. 30
We speculate that dysregulated BK signaling might even explain some of the perplexing observations on COVID‐19. Emerging data suggest that in the United States of America, morbidity and mortality among African Americans has been disproportionately higher compared to other ethnic groups. 31 , 32 , 33 It is possible that African Americans are more affected due to their increased sensitivity to BK, a greater susceptibility to ACE‐inhibitor‐induced angioedema, and a polymorphism (XPNPEP2 C‐2399A) linked to ACE‐inhibitor‐induced angioedema in African American males. 34 , 35 , 36 , 37 In addition, data suggest that respiratory complications are more often seen in males than females. 38 , 39 , 40 , 41 Interestingly, APP activity has been reported to be higher in females irrespective of the XPNPEP2 C‐2399A polymorphism. 37 Furthermore, vasopressors are required to stabilize some patients with COVID‐19 critical illness 17 , 38 ; and, it is well‐known that BK elevation reduces blood pressure. 42 , 43 , 44 A loss of smell and/or taste has been reported by some patients with COVID‐19 45 , 46 , 47 ; and, a loss of smell has been reported in patients with HAE and in persons who take ACE inhibitors. 48 , 49 There are anecdotes of individuals experiencing extreme thirst while having COVID‐19 50 , 51 ; and, elevated BK and ACE inhibitors are associated with increased thirst. 52 However, confounding factors might exist, and, therefore, these observations will need to be evaluated more objectively.
In summary, established and emerging evidence on SARS‐CoV, ACE2, BK and DABK signaling, angioedema, and respiratory distress, has helped us develop a testable hypothesis, which may link dysregulated BK signaling to COVID‐19 respiratory complications. There is a critical need to develop basic and clinical studies to test this hypothesis, since there are approved drugs that might be effective in interrupting the vicious feedback loop that might exist between dysregulated BK signaling and tissue injury.
CONFLICT OF INTEREST
The authors have no conflicts to declare.
AUTHOR CONTRIBUTIONS
J.A. Roche and R. Roche were involved in reviewing the literature and writing this manuscript.
ACKNOWLEDGMENTS
JAR received support in the form of startup funds and laboratory space from Wayne State University, Detroit, MI, USA.
Roche JA, Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID‐19 respiratory complications. The FASEB Journal. 2020;34:7265–7269. 10.1096/fj.202000967
This article was fast‐tracked under a recently instituted interim policy in which the editors may, at their discretion, accept coronavirus‐related manuscripts submitted for the Review, Perspectives and Hypotheses categories without additional review.
REFERENCES
- 1. World Health Organization (WHO) . WHO Health Emergency Dashboard—WHO (COVID‐19) Homepage. Vol. 2020. Webpage, World Health Organization; 2020. [Google Scholar]
- 2. Giwa AL, Desai A, Duca A. Novel 2019 coronavirus SARS‐CoV‐2 (COVID‐19): an updated overview for emergency clinicians. Emergency Medicine Practice. 2020;22:1‐28. [PubMed] [Google Scholar]
- 3. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . Off‐label use of medicines for COVID‐19. Vol. 2020. Webpage, World Health Organization. [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94:e00127‐00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sodhi CP, Wohlford‐Lenane C, Yamaguchi Y, et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des‐Arg(9) bradykinin/BKB1R axis and facilitates LPS‐induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17‐L31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia H. Pulmonary angiotensin‐converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239‐248. [DOI] [PubMed] [Google Scholar]
- 10. Duchene J, Ahluwalia A. The kinin B1 receptor and inflammation: new therapeutic target for cardiovascular disease. Curr Opin Pharmacol. 2009;9:125‐131. [DOI] [PubMed] [Google Scholar]
- 11. Breil I, Koch T, Belz M, Van Ackern K, Neuhof H. Effects of bradykinin, histamine and serotonin on pulmonary vascular resistance and permeability. Acta Physiol Scand. 1997;159:189‐198. [DOI] [PubMed] [Google Scholar]
- 12. Cabrini DA, Calixto JB. Characterization of des‐Arg9‐bradykinin‐induced contraction in guinea‐pig gallbladder in vitro. Eur J Pharmacol. 1997;331:31‐38. [DOI] [PubMed] [Google Scholar]
- 13. Charignon D, Spath P, Martin L, Drouet C. Icatibant, the bradykinin B2 receptor antagonist with target to the interconnected kinin systems. Expert Opin Pharmacother. 2012;13:2233‐2247. [DOI] [PubMed] [Google Scholar]
- 14. Lumry WR, Li HH, Levy RJ, et al. Randomized placebo‐controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST‐3 trial. Ann Allergy Asthma Immunol. 2011;107:529‐537. [DOI] [PubMed] [Google Scholar]
- 15. Rhaleb NE, Rouissi N, Jukic D, et al. Pharmacological characterization of a new highly potent B2 receptor antagonist (HOE 140: D‐Arg‐[Hyp3, Thi5, D‐Tic7, Qic8]bradykinin). Eur J Pharmacol. 1992;210:115‐120. [DOI] [PubMed] [Google Scholar]
- 16. Bas M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE‐inhibitor‐induced angioedema. N Engl J Med. 2015;372:418‐425. [DOI] [PubMed] [Google Scholar]
- 17. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Shamlan F, El‐Hashim AZ. Bradykinin sensitizes the cough reflex via a B2 receptor dependent activation of TRPV1 and TRPA1 channels through metabolites of cyclooxygenase and 12‐lipoxygenase. Respir Res. 2019;20(1):110. 10.1186/s12931-019-1060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zanichelli A, Maurer M, Aberer W, et al. Long‐term safety of icatibant treatment of patients with angioedema in real‐world clinical practice. Allergy. 2017;72:994‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turner P, Dear J, Scadding G, Foreman JC. Role of kinins in seasonal allergic rhinitis: icatibant, a bradykinin B2 receptor antagonist, abolishes the hyperresponsiveness and nasal eosinophilia induced by antigen. J Allergy Clin Immunol. 2001;107:105‐113. [DOI] [PubMed] [Google Scholar]
- 21. Allen TC, Kurdowska A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 2014;138:266‐269. [DOI] [PubMed] [Google Scholar]
- 22. Molinaro G, Duan QL, Chagnon M, et al. Kinin‐dependent hypersensitivity reactions in hemodialysis: metabolic and genetic factors. Kidney Int. 2006;70:1823‐1831. [DOI] [PubMed] [Google Scholar]
- 23. Molinaro G, Cugno M, Perez M, et al. Angiotensin‐converting enzyme inhibitor‐associated angioedema is characterized by a slower degradation of des‐arginine(9)‐bradykinin. J Pharmacol Exp Ther. 2002;303:232‐237. [DOI] [PubMed] [Google Scholar]
- 24. Hudey SN, Westermann‐Clark E, Lockey RF. Cardiovascular and diabetic medications that cause bradykinin‐mediated angioedema. J Allergy Clin Immunol Practice. 2017;5:610‐615. [DOI] [PubMed] [Google Scholar]
- 25. Farmer SG, Wilkins DE, Meeker SA, Seeds EA, Page CP. Effects of bradykinin receptor antagonists on antigen‐induced respiratory distress, airway hyperresponsiveness and eosinophilia in guinea‐pigs. Br J Pharmacol. 1992;107:653‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murugesan P, Jung B, Lee D, Khang G, Doods H, Wu D. Kinin B1 receptor inhibition with BI113823 reduces inflammatory response, mitigates organ injury, and improves survival among rats with severe sepsis. J Infect Dis. 2016;213:532‐540. [DOI] [PubMed] [Google Scholar]
- 27. Ingelheim B. Safety, Tolerability and Pharmacokinetics of Single Rising Oral Doses of BI 113823 Powder in Bottle (PiB) and Tablet in Healthy Male Volunteers. (U.S. Food and Drug Administration, ed); 2014. https://ClinicalTrials.gov/show/NCT02259972.
- 28. Ingelheim B. Safety, tolerability, pharmacokinetics and ‐dynamics of multiple rising oral doses of BI 113823 in patients patients with osteoarthritis of the knee. (U.S. Food and Drug Administration, ed); 2010.https://ClinicalTrials.gov/show/NCT01207973.
- 29. Farkas H, Varga L. Ecallantide is a novel treatment for attacks of hereditary angioedema due to C1 inhibitor deficiency. Clin Cosmet Investig Dermatol. 2011;4:61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saule C, Boccon‐Gibod I, Fain O, et al. Benefits of progestin contraception in non‐allergic angioedema. Clin Exp Allergy. 2013;43:475‐482. [DOI] [PubMed] [Google Scholar]
- 31. Yancy CW. COVID‐19 and African Americans. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 32. Johns Hopkins University and Medicine . Racial Data Transparency—States that have released breakdowns of Covid‐19 data by race. Vol. 2020. Baltimore, MD: Johns Hopkins University and Medicine—Coronavirus Resource Center; 2020. [Google Scholar]
- 33. Moore N. In Chicago, COVID‐19 is hitting the black community hard. Vol. 2020. Washington, DC: National Public Radio (npr); 2020. [Google Scholar]
- 34. Gainer JV, Nadeau JH, Ryder D, Brown NJ. Increased sensitivity to bradykinin among African Americans. J Allergy Clin Immunol. 1996;98:283‐287. [DOI] [PubMed] [Google Scholar]
- 35. Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol. 1999;48:861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor‐associated angioedema. Clin Pharmacol Ther. 1996;60:8‐13. [DOI] [PubMed] [Google Scholar]
- 37. Woodard‐Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ. Sex‐dependent and race‐dependent association of XPNPEP2 C‐2399A polymorphism with angiotensin‐converting enzyme inhibitor‐associated angioedema. Pharmacogenet Genomics. 2010;20:532‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically Ill patients in the seattle region—case series. N Engl J Medicine. 2020. 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walter LA, McGregor AJ. Sex‐ and gender‐specific observations and implications for COVID‐19. Western J Emerg Med. 2020. 10.5811/westjem.2020.4.47536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manolis AJ, Marketou ME, Gavras I, Gavras H. Cardioprotective properties of bradykinin: role of the B2 receptor. Hypertens Res. 2010;33:772‐777. [DOI] [PubMed] [Google Scholar]
- 43. Tom B, Dendorfer A, de Vries R, Saxena PR, Jan Danser AH. Bradykinin potentiation by ACE inhibitors: a matter of metabolism. Br J Pharmacol. 2002;137:276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gavras I. Bradykinin‐mediated effects of ACE inhibition. Kidney Int. 1992;42:1020‐1029. [DOI] [PubMed] [Google Scholar]
- 45. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? Rhinology; 2020. 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 46. American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) . COVID‐19 Anosmia Reporting Tool. Vol. 2020, Alexandria, VA: American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS); 2020. [Google Scholar]
- 47. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. International Forum of Allergy & Rhinology n/a. 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neil‐Dwyer G, Marus A. ACE inhibitors in hypertension: assessment of taste and smell function in clinical trials. J Hum Hypertens. 1989;3(Suppl 1):169‐176. [PubMed] [Google Scholar]
- 49. Perricone C, Agmon‐Levin N, Shoenfeld N, et al. Evidence of impaired sense of smell in hereditary angioedema. Allergy. 2011;66:149‐154. [DOI] [PubMed] [Google Scholar]
- 50. Copaken D. My Whole Household Has COVID‐19. Vol. 2020. Washington, DC: The Atlantic; 2020. [Google Scholar]
- 51. Murray J. “This really hurts”: man shares Covid‐19 experience in video. Vol. 2020. England, UK: The Guardian; 2020. [Google Scholar]
- 52. Cadnapaphornchai MA, Rogachev B, Summer SN, et al. Evidence for bradykinin as a stimulator of thirst. Am J Physiol Renal Physiol. 2004;286(5):F875‐F880. [DOI] [PubMed] [Google Scholar]
- 53. Lim YX, Ng YL, Tam JP, Liu DX. Human coronaviruses: a review of virus‐host interactions. Diseases. 2016;4(3):E26. 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol. 2014;5:296. 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yeung YS, Yip CW, Hon CC, et al. Transcriptional profiling of Vero E6 cells over‐expressing SARS‐CoV S2 subunit: insights on viral regulation of apoptosis and proliferation. Virology. 2008;371:32‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. J Virol. 2010;84:1289‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krahling V, Stein DA, Spiegel M, Weber F, Muhlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J Virol. 2009;83:2298‐2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leeb‐Lundberg LMF, Marceau F, Müller‐Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the Kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27‐77. [DOI] [PubMed] [Google Scholar]
- 59. Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein‐kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6‐38. [DOI] [PubMed] [Google Scholar]
- 60. Maas C. Plasminflammation—an emerging pathway to bradykinin production. Front Immunol. 2019;10:2046. 10.3389/fimmu.2019.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]


