Abstract
Objectives
The emergence of SARS‐CoV‐2 has presented clinicians with a difficult therapeutic dilemma. With supportive care as the current mainstay of treatment, the fatality rate of COVID‐19 is 6.9%. There are currently several trials assessing the efficacy of different antivirals as treatment. Of these, chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) have garnered the most attention.
Methods
In this study, the literature currently available on CQ and HCQ as treatment of COVID‐19 was surveyed using EMBASE, PubMed, Cochrane Library, MedRxiv, and one clinical trial registry. Upon gathering published and preprint trials, risk of bias was assessed using Cochrane Risk of Bias Tool 2.0.
Results
There are currently seven completed clinical trials and 29 registered clinical trials focusing on HCQ or CQ as a therapeutic avenue for COVID‐19. Of these, five of seven trials have shown favorable outcomes for patients using CQ or HCQ and two of seven have shown no change compared to control. However, all seven trials carried varying degrees of bias and poor study design.
Conclusion
There are currently not enough data available to support the routine use of HCQ and CQ as therapies for COVID‐19. Pending further results from more extensive studies with more stringent study parameters, clinicians should defer from routine use of HCQ and CQ. There are several clinical trials currently under way with results expected soon.
Coronaviruses are positive‐sense, single‐stranded enveloped RNA viruses. In December 2019, a novel coronavirus endemic to China was identified as the cause of a series of pneumonia cases in the region of Wuhan. The virus spread rapidly thereafter, resulting in the World Health Organization (WHO) declaring it a pandemic in March 2020. 1 The novel coronavirus was named severe acute respiratory syndrome coronavirus (SARS‐CoV‐2), and the disease caused by the virus named COVID‐19. Current theories suggest a zoonotic origin with genomic analysis showing a close resemblance with two other highly contagious human coronaviruses, MERS‐CoV and SARS‐CoV. 2 As of April 26, 2020, there have been more than 2,900,000 cases reported globally, with more than 206,000 deaths and 860,000 recoveries from COVID‐19, according to Johns Hopkins University. 3
At present, the mainstay of treatment for COVID‐19 thus far has been mainly supportive. Those with nonsevere illnesses (fever, cough, myalgias, etc.) are managed with home care and self‐isolation. Home care includes use of hydration, antipyretics, analgesics, and antitussives as necessary with use of face masks and the maintenance of 6 feet of distance when in the presence of other people. Frequent handwashing and disinfection of frequently touched surfaces is also recommended by the Centers for Disease Control and Prevention. 4 Those with illness proven by positive COVID‐19 screening tests are advised to discontinue home isolation at least 7 days after start of symptoms, and at least 3 days after becoming asymptomatic (resolution of fever and respiratory symptoms). Those who are asymptomatic are asked to self‐isolate for at least 7 days after a positive test result. 5 Those with severe COVID‐19 are admitted into the hospital, where they are managed with oxygen support via high‐flow oxygen or noninvasive positive pressure ventilators. Currently, the WHO recommends against the use of glucocorticoids. 6 Some patients go on to develop acute respiratory distress syndrome requiring intubation with mechanical ventilation in an intensive care unit setting. There has recently been investigation exploring the use of certain antivirals in the treatment of COVID‐19, with clinical trials currently under way measuring their effectiveness. Some of these experimental treatments include remdesivir, chloroquine (CQ)/hydroxychloroquine (HCQ), IL‐6 inhibitors, convalescent plasma, favipiravir, and lopinavir‐ritonavir. Of these, chloroquine/hydroxychloroquine has gained the most media attention after President Donald Trump of the United States urged patients to take it. 7
Chloroquine is used extensively as an antimalarial and immunomodulating agent. HCQ, a derivative of CQ with an extra hydroxyl group, is shown to be less toxic than CQ in animal studies. 8 HCQ is commonly used in rheumatologic conditions, such as systemic lupus erythematosus and rheumatoid arthritis and conditions like porphyria cutanea tarda, Q fever, and malaria. The anti‐inflammatory properties of HCQ are thought to be due to interference of antigen processing in macrophages and antigen‐presenting cells by increasing the pH within intracellular vacuoles and endosomes. 9 Common side effects of the drug include nausea, diarrhea, QTc prolongation, and retinopathy from chronic use. CQ and HCQ have recently gained international attention for their efficacy against SARS‐CoV‐2 in vitro. 10 However, objective clinical data evaluating their use are limited. As of March 30, 2020, the U.S. Food and Drug Administration has issued an emergency use authorization for CQ and HCQ in adolescents and adults hospitalized for COVID who are unable to participate in clinical trials. 11 This study aims to review the literature currently available regarding the clinical use of CQ and HCQ as treatment in COVID‐19 patients in an effort to catalog their recommendations and assess drug efficacy.
Methods
This is a systematic review performed to analyze the current literature to find the role of CQ and HCQ in the treatment of COVID‐19 patients. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were used for this review. 12 This review was not registered on Prospero because data extraction began as soon as clinical trial data were made available due to the urgency of the crisis.
Eligibility Criteria
The studies selected were:
Randomized or nonrandomized clinical trials assessing the efficacy or safety of HCQ or CQ use in patients with COVID‐19.
Participants in the trials could be of any age, in any geographic location.
Published articles, preprint manuscripts, abstracts, letter to the editors, or currently undergoing trials.
Completed between December 1, 2019, and April 26, 2020.
The indiscriminate nature of the eligibility criteria is due to the evolving nature of the pandemic and the limited number of completed clinical trials. The primary outcomes prioritized in this study were mortality, clinical improvement, radiologic improvement, clinical complications, drug adverse events, and negative SARS‐CoV‐2 polymerase chain reaction (PCR) or nasopharyngeal swab posttreatment. However, any outcome analyzed by the studies was also considered.
Electronic search was completed using these databases:
Cochrane Library.
MEDLINE.
EMBASE.
MedRxiv.
Clinical trials that are ongoing were searched in the registry below:
Keywords used for searches in all databases and registry are detailed below:
“Hydroxychloroquine” + “chloroquine” and “COVID 19” + “coronavirus” + “novel coronavirus” + “SARS‐CoV‐2” + “COVID” + “COVID‐19”
No restrictions were placed on search parameters, including status, date, or language. The results of the search are detailed in Figure 1 below.
Figure 1.
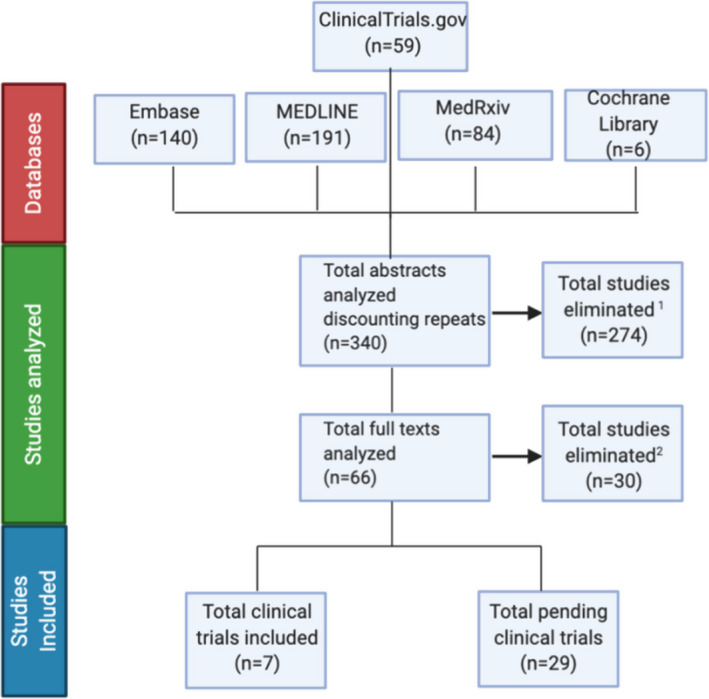
Search results and flow diagram. 1Studies eliminated that were not clinical trials. 2Studies eliminated that were not looking at HCQ/CQ as treatment for COVID‐19.
Screening
The results of the databases and registry were searched and analyzed by two authors independently (MC and JR). Titles and abstracts were screened to isolate clinical trials utilizing HCQ or CQ as the experimental arm. Those that met eligibility requirements were read in full to extract clinical data pertaining to outcomes detailed. Those that included HCQ or CQ specifically as therapeutic agents (rather than as prophylactic agents) were included in this review. Any discrepancies in data collection or extraction were solved by consensus with the help of a third party (JG).
Risk of Bias Assessment
Risk of bias within completed clinical trials was assessed for each study using Cochrane Risk Bias Tool 2.0. 13
Summary Measures and Synthesis of Results
It was not possible to conduct a meta‐analysis given the heterogeneity of the trials included and lack of adequate data availability. Points of heterogeneity that precluded quantitative analysis included study design (some studies were randomized while some were nonrandom), study protocol (some studies were intention‐to‐treat while others were per‐protocol), variability in experimental intervention groups, variability in control intervention groups, deviation from stated intervention (some studies included additional intervention depending on clinical circumstance), and differing primary outcomes. As such, results and data are presented as an integrative qualitative review in a narrative format.
Results
Using the databases listed, initial search on April 9, 2020, and a subsequent search on April 26, 2020, yielded a total of 340 abstracts. Of those, 274 were eliminated because they did not meet the eligibility criteria. Sixty‐six abstracts were further investigated with their full texts analyzed. Of those full texts, 30 were eliminated because they did not use HCQ or CQ as treatment arms but rather as prophylactic agents. The remaining 36 studies were analyzed in full, with data points extracted as per protocol. These studies included seven completed clinical trials, which was composed of three preprint texts, 14 , 15 , 16 two published texts, 17 , 18 , 19 and one letter of declaration 20 of results. They also included 29 ongoing clinical trials. Results and study design from completed clinical trials are detailed in Tables 1 and 2. Data extracted from ongoing clinical trials are detailed in Data Supplement S1 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14005/full). One publication 21 in Chinese was translated to English using Google Translator Web service before review.
Table 1.
Study Design of Completed Clinical Trials Evaluating HCQ/CQ as Treatment for COVID‐19
| Title | Author | Publication Date, Data Collection Dates* | Institution/Country Study Conducted | Design | Inclusion Criteria | Exclusion Criteria | Participants | Intervention | Control | Primary Endpoint(s) | Secondary Endpoint(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial 18 | Gautret et al. | Published 3/20/20, with data collected up to 3/14/20 | University Hospital Institute Méditerrané Infection in Marseille, France | Open‐label, nonrandomized clinical trial, per‐protocol analysis |
|
|
42 patients | HCQ 600 mg D1–D10 ± azithromycin 500 mg LD, 250 mg D2–D5 + standard of care | Standard of care |
|
|
| A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19) 19 | Chen et al. | Published 2/29/20, data collected 2/5/20–2/25/20 | Shanghai Public Health Clinical Center in Shanghai, China | Open‐label, RCT, intention‐to‐treat analysis |
|
|
30 patients | HCQ 400 mg D1–D5 + standard of care | Standard of care (included holding the treatment, and using antivirals if necessary) |
|
|
| Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a 6‐day follow‐up: an observational study 17 | Gautret et al. | Preprint, data collected 3/3/20–3/21/20 | University Hospital Institute Méditerrané Infection in Marseille, France | Open‐label, clinical trial, no information about randomization, intention‐to‐treat analysis |
|
|
80 patients | HCQ 600 mg D1–D10 + azithromycin 500 mg LD, 250 mg D2–D5 | NA |
|
|
| Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial 14 | Chen et al. | Preprint, data collected 2/4/20– 2/28/20 | Renmin Hospital of Wuhan University in Wuhan, China | Double‐blind, RCT, intention‐to‐treat analysis |
|
|
62 patients | HCQ 400 mg D1–D5 + standard of care | Standard of care |
|
|
| Breakthrough chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies 20 | Gao et al. | Published 2/19/20, not stated when collected | 10 hospitals in China in cities of Wuhan, Jingzhou, Guangzhou, Beijing, Shanghai, Chingqing, and Ningbo | Unclear—letter of declaration of results |
|
|
100 patients | CQ 500 mg BID D1–D10 + standard of care | Not listed |
|
|
| Treating COVID‐19 with chloroquine 15 | Huang et al. | Published 4/1/20, data collected 1/27/20–2/15/20 | Fifth Affiliated Hospital of Sun Yat‐sen University in Zhuhai, China | No information about blinding, RCT, intention‐to‐treat analysis |
|
|
22 patients | CQ 500 mg BID D1–D10 + lopinavir/ritonavir 400 mg/100 mg BID D1–D10 | Lopinavir/ritonavir 400 mg/100 mg D1–D10 |
|
|
| Hydroxychloroquine in patients with COVID‐19: an open‐label, randomized, controlled trial 16 | Tang et al. | Preprint, data collected 2/11/20–2/29/20 | 16 Chinese government designated COVID‐19 centers in three provinces (Hubei, Henan, Anhui) | Open‐label, RCT, intention‐to‐treat analysis |
|
|
150 patients |
HCQ 1,200 mg LD D1–D3, 800 mg D4 up to D14 for mild/moderate symptoms HCQ 1,200 mg LD D1–D3, 800 mg D4 up to D21 for severe symptoms + standard of care (included use of antivirals) |
Standard of care (included use of antivirals) |
|
|
Standard of care is bedrest, oxygen supplementation, and supportive care unless otherwise indicated.
CQ = chloroquine; D = day (followed by number); HCQ = hydroxychloroquine; LD = loading dose; RCT = randomized clinical trial; RT‐PCR = reverse transcriptase polymerase chain reaction; TTCR = time to clinical recovery.
Dates are reported as month/day/year.
Table 2.
Results of Completed Clinical Trials Evaluating HCQ/CQ as Treatment for COVID‐19
| Author | Group Design | Intervention | Control | Primary Endpoint(s) | Results | Comments/Problems |
|---|---|---|---|---|---|---|
| Gautret et al. 18 |
HCQ: 26 patients (6 patients lost to follow‐up) Control: 16 patients Grouped into 3 categories: asymptomatic, LRTI, URTI |
HCQ 600 mg D1–D10 ± azithromycin 500 mg LD, 250 mg D2–D5 depending on clinical presentation [6 patients received this tx to prevent bacterial super infection) | Standard of care |
|
HCQ group: 70% (13/20) had negative RT‐PCR on D6 Control group: 12.5% (2/16) had negative RT‐PCR on D6 HCQ + azithromycin group: 100% (6/6) had negative RT‐PCR on D6 |
|
| Chen et al. 19 |
HCQ: 15 patients Control: 15 patients |
HCQ 400 mg D1–D5 | Standard of care (included holding the treatment and using antivirals if necessary) |
|
HCQ group: 86.7% (13/15) had negative RT‐PCR on D7 Median time for temperature normalization: 1 day (95% CI = 0–2 days) Radiologic progression: 5 people Median duration until negative PCR: 4 days (95% CI = 1–9 days) Transient diarrhea and abnormal liver function: 4/15 Control group: 93.3% (14/15) had negative RT‐PCR on D7 Median time for temperature normalization: 1 day (95% CI = 0–3 days) Radiologic progression: 5 people Median duration until negative PCR: 4 days (95% CI = 1–4 days) Transient diarrhea and abnormal liver function: 3/15 |
|
| Gautret et al. 17 |
HCQ: 80 patients Control: None Patients were stratified by: Symptoms—4 patients asymptomatic, 43 patients with URTI, 33 patients with LRTI NEWS—69 patients with low score (0‐4), 4 patients with medium score (5‐6), and 2 patients with high score (>7) |
HCQ 600 mg D1–D10 + azithromycin 500 mg LD, 250 mg D2–D5 | NA |
|
|
|
| Chen et al. 14 |
HCQ: 31 patients Control: 31 patients |
HCQ 400 mg D1–D5 | Standard of care | TTCR—defined as normalized body temperature and cough relief for 72+ hours |
HCQ group: TTCR: Fever length: 2.2 ± 0.4 days,Cough length: 2.0 ± 0.2 days 80.6% (25/31) had improved pneumonia per chest CT Two patients had mild adverse reactions. Control group: TTCR:Fever lengths: 3.2 ± 1.3 days, Cough length: 3.1 ± 1.5 days 54.8% (17/31) had improved pneumonia per chest CT. Four patients progressed to severe illness. |
|
| Gao et. al 20 | Not listed | CQ 500 mg BID D1–D10 | Standard of care |
|
No details given other than CQ is effective in improving all primary endpoints outcomes. |
|
| Huang et al. 15 |
CQ: 10 patients: 3 severe 7 moderate Indinavir/lopinavir: 12 patients: 5 severe 7 moderate |
CQ 500 mg BID D1–D10 + lopinavir/ritonavir 400 mg/100 mg BID D1–D10 | Lopinavir/ritonavir 400 mg/100 mg D1–D10 |
|
CQ group: By D13, 100% (10/10) had negative RT‐PCR CT findings: 100% (10/10) showed CT improvement on D14 Hospital stay: 100% (10/10) discharged by D14 Nine patients had adverse events including vomiting, abdominal pain, nausea, rash, pruritus, cough, SOB Lopinavir/ritonavir: By D14, 91.7% (11/12) had negative RT‐PCR CT findings: 75% (9/12) showed CT improvement on D14 Hospital stay: 50% (6/12) discharged by D14 |
|
| Tang et al. 16 |
HCQ group: 75 patients enrolled 70 patients analyzed Control group: 75 patients enrolled 80 patients analyzed |
HCQ 1,200 mg LD D1–D3, 800 mg D4 up to D14 for mild/moderate symptoms HCQ 1,200 mg LD D1–D3, 800 mg D4 up to D21 for severe symptoms + Standard of care (included use of antivirals) |
Standard of care (included use of antivirals) |
|
HCQ group: RT‐PCR negative D28: 85.4% (95% CI = 73.8%–93.8%) Median time to negative RT‐PCR: 8 days Time to alleviation of clinical symptoms: 19 days Reduction in CRP: 6.98 Absolute change of lymphocytes: 0.062 × 109/L Adverse effects: 30% (21/70) Control RT‐PCR negative D28: 81.3% (95% CI = 71.2%–89.6%, p = 0.34) Median time to negative RT‐PCR: 8 days (p = 0.341) Time to alleviation of clinical symptoms: 21 days Reduction in CRP: 2.72 Absolute change of lymphocytes: 0.008 x 109/L Adverse effects: 8.8% (7/80) Post hoc analysis performed after removal of confounder (antivirals) Alleviation of clinical symptoms: HCQ showed better efficacy hazard ratio: 8.83 (95 CI = 1.09 to 71.3) |
|
Standard of care is bedrest, oxygen supplementation, and supportive care unless otherwise indicated.
CQ = chloroquine; CRP = C‐reactive protein; D‐, Day‐; LD = Loading dose; NEWS = National Early Warning Score; RCT = randomized clinical trial; LRTI = lower respiratory tract infection; RT‐PCR = reverse transcriptase polymerase chain reaction; SOB = shortness of breath; TTCR = Time to clinical recovery; URTI = upper respiratory tract infection.
Risk of bias was calculated for all completed clinical trials included in this review except for Gao et al., 20 because there was no information about study design or data regarding intervention or primary outcome in the publication. Bias assessment for completed clinical trials is included below in Figure 2.
Figure 2.
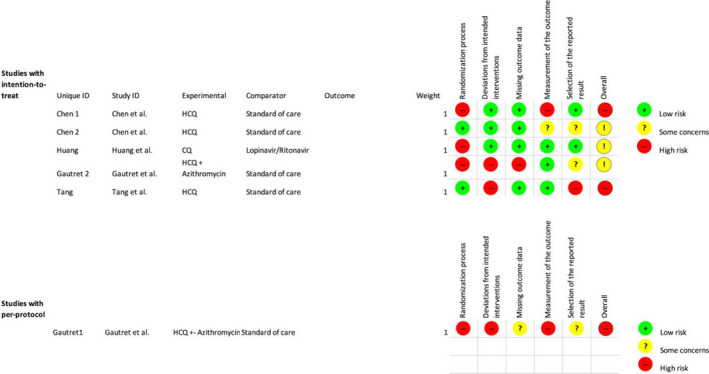
Study bias as per Cochrane Risk Bias Tool 2.013. Chen 1 = Chen et al.; 19 Chen 2 = Chen et al.; 14 Huang: Huang et al.; 15 Gautret 2 = Gautret et al.; 17 Tang = Tang et al.; 16 Gautret 1 = Gautret et al. 18
Discussion
The anti‐inflammatory and antiviral properties of CQ and HCQ have been catalogued in several studies performed in vitro. 21 , 22 , 23 The mechanism of action by which CQ and HCQ exhibit antiviral properties against SARS‐CoV‐2 has not be fully elucidated but presumed to be due to the alkaline nature of the drug, which causes an increase in pH within endosomes in cells, leading to the prevention of viral entry and transport. In addition, CQ has previously shown an ability to block glycosylation of cell surface receptors, disabling the ability of SARS‐CoV‐1 to bind to angiotensin‐converting enzyme 2 receptors, which exist in abundance in human heart, lung, kidney, and intestines. 24 Since SARS‐CoV‐2 is thought to utilize the same mechanism for cell attachment and entry, CQ and HCQ exhibit significant promise in blocking initial viral infection in vitro. Given these promising in vitro results and the overwhelming demands of finding an effective treatment in the face of a rapidly evolving global health emergency, multiple in vivo clinical trials were set in motion from December 2019 to April 2020 to evaluate the efficacy of HCQ and CQ as therapeutic agents in COVID‐19. Our search showed that there are at present seven such clinical trials with published or prepublished results. However, due to poor study design and haphazardly chosen outcomes, the results of these in vivo studies are less convincing than those in vitro.
The first published clinical trials evaluating antiviral activity of CQ in COVID‐19 patients were from China. Gao et al. 20 published the first study in letter format, where they enlisted “more than 100 patients” and found CQ superior to control intervention (which they do not elucidate on) in clinical improvement of pneumonia, improvement of imaging findings, and shortening of disease course. This study prompted the National Health Commission of the People's Republic of China to establish the use of CQ (500 mg BID for 10 days maximum) nationwide in adults with COVID‐19. 25 Despite the promising results, the letter did not include any information about trial design or give any further information about the study results. It did mention that there were a “number of subsequent trials” under way to study the same intervention. Most of these trials were never completed or published, but one (Chen et al. 19 ) published just 10 days later showed that an intervention of HCQ yielded no difference in clinical improvement, imaging findings, and duration of disease course versus supportive care. The study by Chen et al. 19 had its own methodologic limitations, including failure to meet the minimum sample size needed for reliable analysis (i.e., n = 900, study used n = 30) as defined by its own protocol. There was also a lack of uniformity in the interventions, because 12 of 15 patients in the experimental arm and 10 of 15 patients in the control arm also received abidol (an antiviral used in China). Moreover, the study demonstrated substantial risk of bias in randomization because most patients with severe illness were excluded and an exclusion criterion included ability to be excluded based upon “researcher discretion.”
A recently available preprint manuscript of a study conducted by Tang et al. 16 reciprocated similar ambiguous results to that of Chen et al. 19 In this trial, which used a larger sample size (n = 150), the authors compared the antiviral efficacy of high doses of HCQ versus standard of care and reported no significant difference in rate of negative reverse transcriptase–polymerase chain reaction (RT‐PCR) or time to clinical improvement. They assert that HCQ may have more potential in controlling inflammation and preventing disease progression as it led to a significant reduction in C‐reactive protein (6.98 vs. 2.72 in standard of care). A challenge to this assertion is the potential confounding of the results due to use of concomitant antivirals in both treatment groups. The authors acknowledged this potential confounder and report that in a post hoc analysis performed to analyze patients who did not receive antiviral treatment, HCQ provided significant benefit in alleviation of clinical symptoms (hazard ratio = 8.83). This post hoc analysis, however, had a much smaller sample size (n = 28). Additionally, the trial as a whole also poses a significant risk of bias because it did not follow its intention‐to‐treat protocol and moved multiple patients from one intervention arm to another after randomization.
In a separate Chinese study by a different group (Chen et al. 14 ), currently available as a preprint manuscript, HCQ was shown to optimize both time to clinical recovery (TTCR) and radiologic improvement versus supportive care. This study was performed on a group of 62 participants, and both outcomes were significant (p < 0.05). It carries less risk of bias than the previously mentioned studies, because it is the only completed study that both is double‐blinded and follows an intention‐to‐treat protocol. Nevertheless, it still presented with significant methodologic flaws. First, it precluded all critical and severe cases of COVID‐19 “after a doctor's evaluation,” which raises concern for selection bias. Second, the measurement of TTCR only included temperature and cough, foregoing analysis of oxygen exchange data, extubations, renal and hematologic abnormalities, and changes in mental status. It should be noted that neither this study nor the previously published studies included any information about viral load.
The utility of HCQ and CQ has not only been compared to supportive care, but also to other emerging antiviral treatments. In another Chinese study, Huang et al. 15 showed that CQ reduced hospital stay and had greater radiologic improvement of pneumonia compared to lopinavir/ritonavir. While carrying important ramifications, these results are plagued with some of the same pitfalls as previous trials on CQ. Like that of Chen et al., 19 the sample size is small (n = 22) and much of the results were statistically insignificant (p > 0.05). Additionally, there is a significant risk of bias in randomization for this study as patients were on average much older in the CQ group (mean = 53.0 years) compared to the lopinavir/ritonavir group (mean = 41.5 years). Despite these shortcomings, this trial introduces the possibility of multiantiviral treatment of COVID‐19, which is an avenue being assessed by several ongoing clinical trials (Data Supplement S1).
While the early volume in completed clinical trials came from China, much of the international spotlight given to CQ and HCQ has stemmed from the results of a study performed in Marseille, France, by Gautret et al. 18 In this study, HCQ was demonstrated to be efficacious in a cohort of 42 patients by shortening time to virologic clearance as measured by RT‐PCR (p < 0.05). Moreover, HCQ plus azithromycin (which was used in 20 six of participants in the HCQ group to prevent bacterial superinfection) yielded viral clearance in six of six participants (p < 0.05). However, there were major organizational and fundamental problems with this study. First, the study lacked internal validity because there was no blinding or randomization. There was a significant risk of bias in recruitment of participants as all participants in the experimental arm were recruited from the same center, whereas the control arm was composed of patients from multiple centers and patients who denied experimental intervention. Furthermore, the study also did not meet the sample size needed for reliable analysis (n = 48) as per its own protocol. From the patients recruited, six of the patients from the experimental arm (16.7%) were lost to follow‐up or had adverse outcomes that were not included in the results. Additionally, the primary outcome of the study (virologic clearance assessed by viral load in RT‐PCR) was analyzed haphazardly, with PCR not performed on each patient every day and viral load listed for certain patients on certain days but excluded for others. The study did attempt to stratify its data according to initial presentation by layering patients into asymptomatic, lower respiratory tract infection, and upper respiratory tract infection groups, but the number of patients in each group was drastically asymmetric and outcomes were not assessed by group. Given these methodologic limitations, the promising results of this trial come with an asterisk. The authors acknowledge that there needs to be further research with a larger cohort but give their recommendations of using HCQ plus azithromycin. They recognize that the combination of the two drugs confers a potential risk of QTc prolongation and necessitates daily electrocardiogram monitoring for patients. Results from this study have inspired outcries from both international governing bodies and scientific communities alike to create numerous similarly designed trials to assess both HCQ and HCQ plus azithromycin as potential therapeutic avenues (Data Supplement S1).
One such trial was conducted by the very same authors. 17 This new study showed that use of HCQ plus azithromycin improved clinical outcome in 65 of 80 patients (p‐value not listed). This study nevertheless contained several design flaws, similar to its predecessor. The most significant of these flaws is the lack of a control intervention. All 80 patients received HCQ plus azithromycin with none receiving supportive care. Six of the patients included were also patients from the previous study who had already received HCQ plus azithromycin. Additionally, the decision to discharge patients was based on a viral RT‐PCR cycle threshold value, but the value was changed three times during the experiment. Despite these shortcomings, this study seems to have been an intention‐to‐treat protocol unlike the authors' previous study, and the authors acknowledge the need for further investigation.
At present, of the seven completed clinical trials evaluating CQ or HCQ efficacy in treatment of COVID‐19, five show that the drugs improve clinical outcome and two show no difference between the drugs and supportive care. However, all seven trials have serious methodological flaws that necessitate further investigation. There are currently several trials under way with more regimented study designs to assess safety and efficacy of these drugs (Data Supplement S1). Although the outcomes of these studies may not be available for quite some time, preliminary findings from select clinical trials and retrospective cohort studies are becoming available in preprint format. One such study, 26 a retrospective cohort study completed using 368 patients in Veterans Affairs centers in the United States concluded that the mortality was higher in HCQ plus azithromycin compared to supportive care (hazard ratio = 2.61, 95% confidence interval [CI] = 1.10 to 6.17, p = 0.03). CQ did not fare much better as another study performed in Brazil 27 (double blind, randomized clinical trial) revealed that high doses of CQ (600 mg BID for 10 days) conferred a higher fatality rate (27%, 95% CI = 17.9% to 38.2%) compared to supportive care. Neither study was included in data and results because they did not match the eligibility criteria (not a clinical trial or trial not completed).
Given the low cost, relatively safe side effect profile, and wide availability of CQ and HCQ compared to other antivirals currently being tested in clinical trials there is a dire need for more evidence for their use. Thus far, there is not sufficient clinical evidence to support the routine use of HCQ or CQ in treatment of COVID‐19. Some data even suggest that they confer a higher fatality rate than control. There must be more robust clinical trials to prove the benefit of these drugs before they are used routinely.
Limitations
The limitations of this review include a small sample of eligible clinical trials (n = 7) and indiscriminate eligibility criteria. Given the evolving nature of COVID‐19, there will be more available clinical trial data with more robust study design and data points to compare to in the coming months.
Conclusion
This rapid systematic review has identified seven different completed clinical trials evaluating the efficacy of hydroxychloroquine or chloroquine as therapy for COVID‐19. The results of the trials show that hydroxychloroquine or chloroquine is efficacious compared to supportive care and to lopinavir/ritonavir in treatment of COVID‐19. However, all the studies analyzed posed significant risk for bias and had significant methodologic flaws. As such, there is still a lack of clinical evidence to support therapeutic use of hydroxychloroquine or chloroquine. There are currently several randomized clinical trials under way with more stringent study design and a greater number of participants, so pending their results, clinicians should defer from the routine use of chloroquine or hydroxychloroquine for COVID‐19.
Supporting information
Data Supplement S1. Ongoing Clinical Trials Evaluating Use of HCQ/CQ as Treatment for COVID‐19.
Academic Emergency Medicine 2020;27:493–504.
The authors have no relevant financial information or potential conflicts of interest to disclose.
Author contributions: MC—study conception, design, acquisition of data, analysis, interpretation, drafting of manuscript, and critical revision of manuscript; JR—study conception, acquisition of data, analysis, interpretation, and critical revision of manuscript; JG—study conception, design, and critical revision of manuscript.
References
- 1. WHO Director‐General's Opening Remarks at the Media Briefing on COVID‐19 – 11 March 2020. World Health Organization. Available at: https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020. Accessed Mar 11, 2020. [Google Scholar]
- 2. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong E, Du H, Gardner L. Interactive web‐based dashboard to track COVID‐19 in real time. Lancet 2020;20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Disposition of Non‐Hospitalized Patients with COVID‐19. Centers for Disease Control and Prevention. Available at: www.cdc.gov/coronavirus/2019‐ncov/hcp/disposition‐in‐home‐patients.html. Accessed Apr 10, 2020. [Google Scholar]
- 5. Return‐to‐Work Criteria for Healthcare Workers . Centers for Disease Control and Prevention. Available at: www.cdc.gov/coronavirus/2019‐ncov/hcp/return‐to‐work.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019‐ncov%2Fhealthcare‐facilities%2Fhcp‐return‐work.html. Accessed Apr 13, 2020.
- 6. Management of Patients with Confirmed 2019‐NCoVInterim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19) . Centers for Disease Control and Prevention. Available at: www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html. Accessed Apr 6, 2020. [Google Scholar]
- 7. Grady D, Kannapell A.Trump Urges Coronavirus Patients to Take Unproven Drug. The New York Times. Available at: www.nytimes.com/2020/04/04/health/coronavirus‐drug‐trump‐hydroxycholoroquine.html. Accessed Apr 5, 2020. [Google Scholar]
- 8. McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med 1983;75:11–8. [DOI] [PubMed] [Google Scholar]
- 9. Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum 1993;23(2 Suppl 1):82–91. [DOI] [PubMed] [Google Scholar]
- 10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐NCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinton DM. Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease. US FDA. Available at: www.fda.gov/media/136534/download. Accessed Mar 28, 2020. [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:4898. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. MedRxiv [preprint]. Available at: www.medrxiv.org/content/10.1101/2020.03.22.20040758v3. [Google Scholar]
- 15. Huang M, Tang T, Pang P, et al.Treating COVID‐19 with Chloroquine. J Mol Cell Biol 2020. 10.1093/jmcb/mjaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID‐19: An Open‐label, Randomized, Controlled Trial. MedRxiv [preprint]. Available at: www.medrxiv.org/content/10.1101/2020.04.10.20060558v1. Accessed Jan 1, 2020. [Google Scholar]
- 17. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis 2020;34:101663. 10.1016/j.tmaid.2020.101663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐ 19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). Zhejiang Da Xue Xue Bao Yi Xue Ban 2020. Available at: www.zjujournals.com/med/EN/10.3785/j.issn.1008‐9292.2020.03.03. Accessed Mar 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao J, Tian Z, Yang X, et al. Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72–3. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang M, Wang M, Cao R, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐NCoV) in vitro. Cell Res 2020;30:369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine Is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Health Commission of the People's Republic of China . Interpretation of COVID‐19 Treatment Guidelines (6th version). Available at: http://www.gov.cn/zhengce/2020‐02/19/content_5480958.htm. Accessed Apr 17, 2020 [in Chinese].
- 26. Magagnoli J. Outcomes of hydroxychloroquine usage in united states veterans hospitalized with Covid‐19. MedRxiv [preprint]. Available at: www.medrxiv.org/content/10.1101/2020.04.16.20065920v1.full.pdf. Accessed Apr 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borba S, Mayla G. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS‐CoV‐2) infection: preliminary safety results of a randomized, double‐blinded, Phase IIb clinical trial (CloroCovid‐19 Study). MedRxiv [preprint]. Available at: www.medrxiv.org/content/10.1101/2020.04.07.20056424v2.full.pdf. Accessed Apr 11, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Ongoing Clinical Trials Evaluating Use of HCQ/CQ as Treatment for COVID‐19.


