Abstract
Few studies reported the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected patients with completely asymptomatic throughout the disease course. We investigated the epidemiological and clinical features of patients infected by SARS‐CoV‐2 without any symptoms. Patients with confirmed SARS‐CoV‐2 infection were retrospectively recruited. The demographic characteristics, clinical data, treatment, and outcomes of SARS‐CoV‐2 infected patients without any symptoms were analyzed. Fifteen (4.4%) of 342 SARS‐CoV‐2 infected patients did not develop any symptom during the course of the disease. The median time from exposure to diagnosis was 7.0 days (interquartile range [IQR]: 1.0‐15.0 days). Of the 15 patients, 14 patients were diagnosed by tested positive for SARS‐CoV‐2 in throat swabs, while one patient was only tested positive for SARS‐CoV‐2 in anal swabs. During hospitalization, only 1 (6.7%) patient developed lymphopenia. Abnormalities of chest computed tomography examinations were detected in 8 (53.4%) patients on admission. As of 8 March 2020, all patients have been discharged. The median time of SARS‐CoV‐2 tested negative from admission was 7.0 days (IQR: 4.0‐9.0 days). Patients without any symptoms but with SARS‐CoV‐2 exposure should be closely monitored and tested for SARS‐CoV‐2 both in anal and throat swabs to excluded the infection. Asymptomatic patients infected by SARS‐CoV‐2 have favorable outcomes.
Keywords: asymptomatic, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection emerged in Wuhan, China, and rapidly spread throughout the world. 1 , 2 As of 13 March 2020, a total of 132 536 confirmed patients with coronavirus disease 2019 (COVID‐19) have been reported in 123 countries with 4947 deaths. 3 The epidemiologic, clinical, and radiologic characteristics of COVID‐19 have been described by several studies. 1 , 4 , 5 Guan et al, 5 analyzed the clinical characteristics of 1099 patients with laboratory‐confirmed COVID‐19 and found that the COVID‐19 has a wide spectrum of severity. The clinical spectrum of COVID‐19 ranges from mild to critically ill cases with fatal outcomes. 5 Previous studies have only described the general epidemiological and clinical findings of patients of COVID‐19. Recently, several studies have reported asymptomatic cases of SARS‐CoV‐2 infection. 6 , 7 , 8 , 9 However, these asymptomatic cases were followed for a very short period. Specific information that characterizes asymptomatic patients remains unknown. As reported by the previous study, fever as one of the dominant symptoms of COVID‐19 was identified in only 43.8% of the patients on presentation, while 88.7% of patients developed a fever after hospitalization indicating that some asymptomatic COVID‐19 cases before admission may develop symptoms during the hospitalization. 5 However, few studies have reported SARS‐CoV‐2 infected patients with completely asymptomatic throughout the disease course.
In this study, we investigated the epidemiological and clinical features of patients infected by SARS‐CoV‐2 with completely asymptomatic throughout the disease course.
2. METHODS
2.1. Patients
Patients with confirmed SARS‐CoV‐2 infection were retrospectively recruited from 10 designated hospitals in 10 cities of Jiangsu province, China (Xuzhou, Lianyungang, Suqian, Huai'an, Yancheng, Nantong, Taizhou, Yangzhou, Changzhou, and Suzhou) from 18 January 2020 to 26 February 2020. All confirmed patients were diagnosed according to the criterion of the World Health Organization (WHO) interim guidance. 10 Patients with completely asymptomatic throughout the disease course were included for the final analysis.
The study was approved by the institutional ethics board of hospitals, with a waiver of informed consent.
2.2. Data collection
The medical records of confirmed cases were reviewed by at least two healthcare staff in each hospital. The demographic characteristics, comorbidities, exposure history, symptoms, laboratory test results, radiological data, treatment, and outcomes were collected. The data were cross‐checked by researchers to avoid errors. Unclear information of patients especially for the symptoms of the patients was further clarified through contacting the specific clinicians directly who were responsible for the treatment of patients. The criteria for discharge of patients was according to the guidelines for the diagnosis and treatment of novel coronavirus infection by the Chinese National Health Commission (Trial Version 5). 11
All patients were confirmed by throat swab samples or anal swab samples detecting by a real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) according to the protocol by the WHO. 12
2.3. Statistical analysis
We expressed the continuous variables as median (interquartile range [IQR]). The categorical data were presented as the counts (percentages). The SPSS version 22.0 software (SPSS Inc, Chicago, IL) was applied for all analyses.
3. RESULTS
3.1. Demographic and epidemiologic characteristics
A total of 342 hospitalized patients during the 18 January 2020 to 26 February 2020 who were confirmed as SARS‐CoV‐2 infection were identified. Three hundred twenty‐three patients had at least one symptom during the disease were excluded. Three children under 3 years old were also excluded considering the possibility that they could not clearly express their symptoms. One asymptomatic patient who was still on admission was also excluded. Finally, 15 patients who were completely asymptomatic throughout the disease course were included for the final analysis. Of the 15 patients, 14 patients were diagnosed by positive SARS‐CoV‐2 in throat swab samples, while one patient was only tested positive for SARS‐CoV‐2 in anal swab samples. The median age of the patients was 27.0 (IQR: 17.0‐36.0) years (Table 1). Two patients were over 60 years old. Ten (66.7%) of patients were men. Four (36.7%) patients were children or adolescents. Three (20.0%) patients had at least one coexisting illness such as hypertension (1 [6.7%]) and chronic liver diseases (2 [13.3%]).
Table 1.
Demographic and epidemiologic characteristics of study population
| Variables (n [%] or median [IQR]) | n = 15 |
|---|---|
| Age, y | 27.0 (17.0, 36.0) |
| ≤60 | 13 (86.7) |
| >60 | 2 (13.3) |
| Male | 10 (66.7) |
| Comorbidities | |
| Hypertension | 1 (6.7) |
| Diabetes | 0 |
| Chronic liver diseases | 2 (13.3) |
| Smoking history | 1 (6.7) |
| Exposure history | |
| Contact with suspected or confirmed patients | 14 (93.3) |
| Contacted with people from Wuhan or non‐Wuhan areas of Hubei province | 3 (20.0) |
| Visited Wuhan or non‐Wuhan areas of Hubei province | 2 (13.3) |
| No contact history | 0 |
| Time from exposure to admission | 8.0 (1.0, 15.0) |
| Time from exposure to diagnosis | 7.0 (1.0, 15.0) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
No patients had direct exposure to Huanan Seafood Market. Fourteen (93.3%) patients had known contact with confirmed cases. One patient visited Fuyang city, where over one hundred confirmed cases were reported. All the asymptomatic patients with SARS‐CoV‐2 infection in our study were identified by the SARS‐CoV‐2 screening due to the contact history with suspected or confirmed cases of COVID‐19. The median time from exposure to diagnosis and from exposure to admission was 7.0 days (IQR: 1.0‐15.0 days) and 8.0 days (IQR: 1.0‐15.0 days), respectively.
3.2. Laboratory and radiologic abnormalities
No patient developed any symptom at onset of illness and during the hospitalization. No patients showed leukopenia and one patient showed lymphopenia (6.7%) on admission. Two (14.3%) of 14 patients had elevated serum C‐reactive protein level (CRP; ≥10 mg/L). Eleven (84.6%) of 13 patients presented normal serum procalcitonin on admission (Table 2). A total of 13 (20.0%) patients had increased levels of alanine aminotransferase (ALT) and 6 (40.0%) patients showed increased lactic dehydrogenase (LDH). Two (13.3%) patients had decreased serum albumin (ALB) level. One (7.7%) of 13 patients had elevated D‐dimer level (Figure 1).
Table 2.
Laboratory and radiology findings of study population
| Variables (n [%] or median [IQR]) | n = 15 |
|---|---|
| Laboratory tests | |
| WBC (×109/L) | 6.3 (4.8, 8.1) |
| Decreased | 0 |
| Neutrophils (×109/L) | 3.0 (2.7, 4.6) |
| Decreased | 1 (6.7) |
| Lymphocytes (×109/L) | 2.3 (1.7, 3.4) |
| Decreased | 1 (6.7) |
| Hb, g/L | 138.0 (131.0, 162.0) |
| Decreased | 0 |
| PLT (×109/L) | 214.0 (142.0, 277.0) |
| Decreased | 1 (6.7) |
| ALT, U/L | 31.0 (15.0, 40.0) |
| Increased | 3 (20.0) |
| LDH, U/L | 195.0 (166.0, 388.0) |
| Increased | 6 (40.0) |
| ALB, g/L | 44.8 (43.0, 48.2) |
| Decreased | 2 (13.3) |
| Cr, μmol/L | 73.0 (50.1, 92.0) |
| Increased | 1 (6.7) |
| Glu, mmol/L | 4.9 (4.6, 5.2) |
| Increased | 0 |
| PT (s) a | 12.6 (12.0, 13.1) |
| Increased | 0 |
| D‐dimer, mg/L b | 0.2 (0.1, 0.3) |
| Increased | 1 (7.7) |
| PCT >0.05 ng/mL c | 2 (15.4) |
| CRP >10 mg/L d | 2 (14.3) |
| Chest CT | |
| No pneumonia | 7 (46.7) |
| Unilateral pneumonia | 4 (26.7) |
| Bilateral pneumonia | 4 (26.7) |
| Ground‐glass opacity | 6 (40.0) |
Abreviations: ALB, albumin; ALT, alanine transaminase; Cr, creatinine; CRP, C‐reactive protein; Glu, glucose; Hb, hemoglobin; IQR, interquartile range; LDH, lactate dehydrogenase; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; WBC, white blood cells.
Available for 12 patients.
Available for 13 patients.
Available for 13 patients.
Available for 14 patients.
Figure 1.

Flow diagram describing the selection of the study population
During hospitalization, no patient presented leucopenia, and only 1 (6.7%) patient developed lymphopenia. Four (26.7%) patients presented an elevated ALT level and 6 (40.0%) patients showed increased LDH level. The peak values of ALT and LDH were 106 U/L and 504 U/L, respectively. Three patients had decreased ALB levels. Elevated serum CRP (≥10 mg/L) was observed in 6 (40.0%) patients (Table 3).
Table 3.
Dynamic change of laboratory parameters during the hospitalization
| Variables (n [%]) | n = 15 |
|---|---|
| WBC (×109/L) | |
| Decreased | 0 |
| Neutrophils (×109/L) | |
| Decreased | 1 (6.7) |
| valley value | 1.43 |
| Lymphocytes (×109/L) | |
| Decreased | 1 (6.7) |
| valley value | 0.87 |
| ALT, U/L | |
| Increased | 4 (26.7%) |
| peak value | 106 |
| LDH, U/L | |
| Increased | 6 (40) |
| peak value | 504 |
| ALB, g/L | |
| Decreased | 3 (20) |
| valley value | 31.6 |
| PCT, ng/mL | |
| Increased | 0 |
| CRP, mg/L | |
| Increased | 6 (40) |
| peak value | 51.17 |
Abbreviations: ALB, albumin; ALT, alanine transaminase; Cr, creatinine; CRP, C‐reactive protein; Glu, glucose; Hb, hemoglobin; LDH, lactate dehydrogenase; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; WBC, white blood cells.
Abnormalities of chest computed tomography (CT) examinations were detected in 8 (53.4%) patients on admission. Of the 8 patients, 4 (50.0%) had bilateral pneumonia and 6 (75.0%) had ground‐glass opacity (Table 2). Representative chest CT images of a 70‐year‐old man on admission were presented in Figure 2. The CT images showed bilateral ground‐glass opacities in both lungs (Figure 2).
Figure 2.
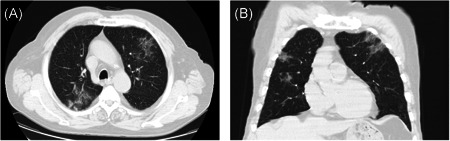
Unenhanced chest CT images of a 70‐year‐old man infected by 2019 novel coronavirus. CT, computed tomography
3.3. Complications, treatment, and outcomes
Two (13.3%) patients received oxygen therapy during hospitalization. No patients received noninvasive mechanical ventilation. Thirteen (86.7%) patients were treated with antiviral therapy (interferon α‐2b, 60.0%; arbidol, 53.3%; lopinavir/ritonavir, 20.0%). Four (26.7%) patients received empirical antibiotic treatment. No patient was given corticosteroids or gamma globulin treatment. No complications were observed in these patients. As of 8 March 2020, all patients have been discharged. The median duration of hospitalization was 11.0 days (IQR: 8.0‐15.0 days). The median time of SARS‐CoV‐2 tested negative from admission was 7.0 days (IQR: 4.0‐9.0 days; Table 4).
Table 4.
Treatment and outcomes of study population
| Variables (n [%] or median [IQR]) | n = 15 |
|---|---|
| Treatment | |
| Oxygen therapy | 2 (13.3) |
| Antiviral therapy | 13 (86.7) |
| Atomized inhalation of interferon α‐2b | 9 (60.0) |
| Arbidol | 8 (53.3) |
| Lopinavir/ritonavir | 3 (20.0) |
| Antibiotic therapy | 4 (26.7) |
| Corticosteroid | 0 |
| Gamma globulin | 0 |
| Outcome | |
| Severe illness | 0 |
| Admission to ICU | 0 |
| Death | 0 |
| Time from hospitalization to negative for SARS‐CoV‐2, d | 7.0 (4.0, 9.0) |
| Duration of hospitalization, d | 11.0 (8.0, 15.0) |
Abbreviations: ICU, intensive care unit; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
4. DISCUSSION
We report 15 patients infected by SARS‐CoV‐2 without any symptoms during the disease course. In our study, 15 (4.4%) of 342 patients did not develop any symptoms during the disease course, indicating that asymptomatic patients with SARS‐CoV‐2 are uncommon. The median age of the patients was 27.0 years and 4 (36.7%) patients were children or adolescents. Previous studies have suggested that SARS‐CoV‐2 is more likely to infect elder adult with underlying diseases. 4 , 13 However, our study provides evidence that young patients infected by SARS‐CoV‐2 without any symptoms should also be monitored. Fourteen of patients in our study had known contact history of confirmed cased and one patient visited Wuhan, suggesting that patients with exposure history to SARS‐CoV‐2 should be screening SARS‐CoV‐2 even though they did not present any symptoms such as fever and cough.
Although the patients were asymptomatic, 10 patients presented laboratory abnormalities. The most common laboratory abnormalities on admission were increased CRP, ALT, and LDH levels. Thus, although patients with COVID‐19 do not present any symptom, laboratory abnormalities were common. Chest CT plays an important role in the initial diagnosis of COVID‐19. 14 Eight (53.4%) patients had abnormal chest CT images on admission in our study indicating that chest CT examination is suggested as an important tool for SARS‐ CoV‐2 infection diagnosis for patients without any symptom. Thus, the patient's epidemiological history, imaging characteristics, and laboratory tests are all important for the diagnosis of SARS‐CoV‐2 infection.
No specific treatment is available for COVID‐19. Thirteen of the patients received antiviral therapy. Thirteen (86.7%) patients received antiviral therapy (interferon α‐2b, 60.0%; arbidol, 53.3%; lopinavir/ritonavir, 20.0%). However, the necessity and benefit of the antiviral agents for asymptomatic COVID‐19 patients is not yet clear.
As of 8 March, all the patients were discharged with the median duration of hospitalization of 11.0 days. No patients developed serious complications. Thus, the clinical outcomes of asymptomatic patients are favorable. However, the median time of SARS‐CoV‐2 tested negative from admission was 7.0 days (IQR: 4.0‐9.0 days) in our study. One patient was tested positive for SARS‐CoV‐2 only in anal swab samples. Given transmission of SARS‐CoV‐2 from asymptomatic carriers have been reported, 6 , 7 asymptomatic patients infected SARS‐CoV‐2 should be isolated to prevent the transmission of this highly contagious disease.
This study was limited by the small sample size with only 15 patients with confirmed COVID‐19 were included. Moreover, although the SARS‐Cov‐2 nucleic acid RT‐PCR test is recognized as the standard method for diagnosis of SARS‐CoV‐2 infection, high false‐negative rates reported. Thus, asymptomatic patients might be underdiagnosed. 15
We described the epidemiological and clinical features of patients infected by SARS‐CoV‐2 with completely asymptomatic throughout the disease course. Our study indicated that asymptomatic patients infected by SARS‐CoV‐2 might have favorable clinical outcomes. Patients who have a contact history of COVID‐19 should be monitored and tested for SARS‐CoV‐2 in anal and throat swabs to rule out infection, even if they are asymptomatic.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Concept and design: LL, CW, and CZ. Drafting of the manuscript: TX, RH, and JW; Critical revision of the manuscript for important intellectual content: RH, CW, LL, and CZ. Statistical analysis: LZ and JW. Supervision: LL and CW. Acquisition, analysis, or interpretation of data: TX, RH, JW, LZ, JC, BZ, HZ, KC, and HS. All authors reviewed and approved the final version.
ACKNOWLEDGMENT
This study was supported by the Fundamental Research Funds for the Central Universities (no. 14380459).
Xu T, Huang R, Zhu L, et al. Epidemiological and clinical features of asymptomatic patients with SARS‐CoV‐2 infection. J Med Virol. 2020;92:1884–1889. 10.1002/jmv.25944
Tianmin Xu, Rui Huang, Li Zhu, and Jian Wang contributed equally.
Contributor Information
Chuanwu Zhu, Email: zhuchw@126.com.
Chao Wu, Email: dr.wu@nju.edu.cn.
Longgen Liu, Email: ssewllg@163.com.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao Y, Torok ME. Taking the right measures to control COVID‐19 [published online Mar 5]. Lancet Infect Dis. 2020;20:523–524. 10.1016/S1473-3099(20)30152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Novel Coronavirus (COVID‐19) Situation [published online March 15, 2020]. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200315‐sitrep‐55‐covid‐19.pdf?sfvrsn=33daa5cb_8
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China [published online Feb 28]. N Engl J Med. 2020;382:1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20:410‐411 [published online Feb 19]. 10.1016/S1473-3099(20)30114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19 [published online Feb 21]. JAMA. 2020;323:1406. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany [published online March 5]. N Engl J Med. 2020;382:970‐971. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 Infection in Beijing [published online Feb 27]. J Infect. 2020;80:401‐406. 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/publications‐detail/clinical‐managementof‐severe‐acute‐respiratory‐infection‐when‐novelcoronavirus‐(ncov)‐infection‐is‐suspected. Accessed Jan 31, 2020.
- 11. National Health Commission . Guidelines for the Diagnosis and Treatment of coronavirus disease 2019 (COVID‐19) by the National Health Commission. http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml. Accessed Feb 16, 2020.
- 12. World Health Organization . Laboratory diagnostics for novel coronavirus. 2020. (https://www.who.int/health‐topics/coronavirus/laboratory‐diagnostics‐for‐novel‐coronavirus. Accessed Feb 6, 2020.
- 13. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China [published online Feb 7]. JAMA. 2020;323:1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong Y, Sun D, Liu Y, et al. Clinical and high‐resolution CT features of the COVID‐19 infection: comparison of the initial and follow‐up changes [published online March 3]. Invest Radiol. 2020;55:332–339. 10.1097/RLI.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis [published online Feb 27, 2020]. J Med Virol. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]


