Abstract
The severe acute respiratory syndrome coronavirus 2 (COVID‐19) pandemic has placed an unprecedented challenge on healthcare systems across the globe. Rapid assessment of the cardiorespiratory function to monitor disease progression and guide treatment is essential. Therefore, we have designed the COVID‐US: a simplified cardiopulmonary ultrasound approach to use in suspected and confirmed COVID‐19 patients, to aid front‐line health workers in their decision‐making in a surge crisis.
Keywords: COVID 19, ultrasound, intensive care
The coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has placed an unprecedented challenge on healthcare systems across the globe. The initial data from Wuhan, China, suggest that approximately 20% of patients require intensive care unit (ICU) admission and 2–4% die due to the illness. The mortality rates exceeding 50% among patients admitted to the intensive care units were now reported from Europe and the United States of America. Viral pneumonia and acute respiratory distress syndrome (ARDS) develops in most of these patients, and a proportion of patients also develop acute heart failure later in the course of the critical illness.1 Rapid assessment of the cardiorespiratory function is therefore essential to monitor disease progression and guide treatment.
Several reasons suggest that cardiopulmonary ultrasound may offer benefit in managing COVID‐19 patients.
Lung ultrasonography features of pneumonia/pneumonitis/ARDS in COVID‐19 are related to the stages of disease and the severity of lung injury.2 It is well known that lung ultrasound is more sensitive and better than chest X‐ray in identifying complications like pneumothorax, effusion and interstitial oedema and its sensitivity is comparable to chest computerised tomography (CT) scan for diagnosing these pathologies.
Echocardiography is standard in investigating circulatory dysfunction contributing to shock in ICU. About 7% of hospitalised patients and about 22% of ICU patients with COVID‐19 are reported to develop acute myocardial injury.3 Early identification of such cardiac involvement may help to guide therapy and titrate levels of support.
Transporting COVID‐19 patients in hospitals for radiology investigation is complicated, given the respiratory and haemodynamic instability and the risk of staff exposure and nosocomial transmission.
The major surge in ICU admissions of COVID‐19 patients will place undue strain on the resources and time available for investigations. Rapid assessment that identifies problems and minimises staff exposure is ideal.
Therefore, we have designed the COVID‐US: a simplified cardiopulmonary ultrasound approach touse in suspected and confirmed COVID‐19 patients to aid front‐line health workers in their decision‐making. With the growing understanding that this unique viral illness involves multiple organs, we believe it is necessary to investigate the cardiopulmonary system on regular basis for timely treatments. Previously, some excellent generic protocols were developed to aim comprehensive assessment of pulmonary and cardiac pathology in critically ill patients.4, 5 However, there are no other existing ultrasound protocols designed for specifics of COVID‐19 which would include both the lung and heart, making the proposed protocol novel to date.
We suggest that emergency, critical care and anaesthesiology clinicians previously trained in both heart and lung ultrasound would be the most suitable users for such a protocol. However, we have not limited its use to any specific craft groups due to the variability in situation within each local setting. Cardiologists and sonographers with lung ultrasound experience, or respiratory physicians with cardiac ultrasound training could also benefit from this protocol in treating the COVID‐19 cases. It is feasible to complete the scan within 5–10 min and repeat as necessary. Practitioners utilising the protocol shall fully understand the pitfalls and limitations of each measurement and the entire study as a whole. This simplified approach is not a replacement for more complex, previously recommended basic and complete investigations. Importantly, it must be applied within individual clinical context to assist in crude therapeutic guidance at the time of major limitations in healthcare resources and time imperative.
Scanning protocol and Image acquisition
The 6 points to be scanned are described below and illustrated in Figure 1.
Figure 1.
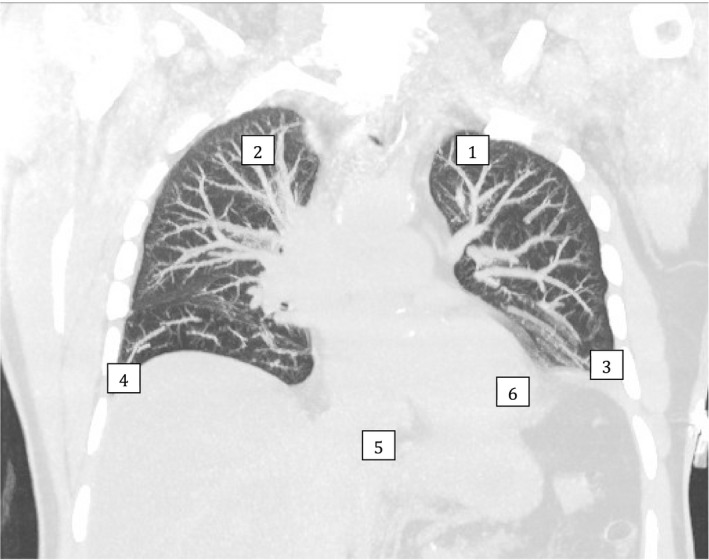
COVID‐US six point scan protocol.
Point 1: Left 2nd intercostal space along midclavicular line. In prone patient, this point is replaced by left paravertebral area in the 2nd intercostal space.
Point 2: Right 2nd intercostal space along midclavicular line. In prone patient, this point is replaced by right paravertebral area in the 2nd intercostal space.
Point 3: Left costophrenic angle.
Point 4: Right costophrenic angle.
Point 5: Subcostal views for inferior vena cava (IVC). In prone patient, this point is replaced by the liver window.
Point 6: Apical view of heart: Left and right ventricular function. This view is often not available in fully proned patient.
Equipment and settings
Ultrasound machine with cardiac and lung package, capable of 2D, M‐mode and pulse wave (PW) Doppler modalities.
Use phased‐array (cardiac) probe.
-
Lung setting for point 1–4: For details of how to perform lung ultrasound, please refer to the previously published guideline.6
-
○
Probe orientation is perpendicular to the ribs with the marker directed cephalad.
-
○
Depth: in point 1 and 2, depth set to disappearance of the second A‐Lines, or 3 times of the ‘skin to pleural’ distance. In point 3 and 4, increase depth allowing full visualisation of effusion and consolidation if any. It is important to angle the probe anteriorly and posteriorly to detect pathologies, which has been reported earlier in the disease.7
-
○
Gain: to allow visualisation of the second A‐Lines or structures at three times the ‘skin to pleural’ distance.
-
○
-
Cardiac settings for points 5–6: For details of how to perform echocardiography, please refer to the previously published guidelines.8
-
○
Clear imaging of IVC: patient is supine, and probe orientation is with anterior tilt and directed towards the left clavicle with marker pointed to the left side of the patient. Once subcostal 4‐chamber view of the heart is obtained, probe is rotated counterclockwise keeping right atrium in the middle of the sector until longitudinal view of the IVC becomes apparent. Another way of visualising the IVC is through the liver window when subcostal view is not possible. Place M‐mode cursor just distal to the hepatic vein‐IVC junction, perpendicular to the IVC. Measure the maximum and minimum diameter in quiet breathing.
-
○
Use apical four chamber for visual estimation of left ventricular (LV) function (care should be taken to minimise foreshortening of the LV). Use apical five‐chamber view. To obtain the left ventricular outflow tract (LVOT) velocity‐time integral (VTI), position the pulsed‐wave Doppler (PWD) range gate in the middle and in‐line with the LVOT, approximately 0.5 cm below the aortic valve. Trace the dense line of the VTI envelope.
-
○
Use apical four‐chamber view, avoiding foreshortening to make accurate visual estimation of right ventricular function. Then, obtain tricuspid annular plane systolic excursion (TAPSE) measurement. Position M‐mode cursor over the medial tricuspid annulus. Measure the distance from the bottom to the top of the continuous brighter line corresponding to the tricuspid annulus.
-
○
Ultrasound findings to disease severity and treatments
All images in this publication are obtained from suspected or confirmed COVID‐19 patients following written informed consent. A colour‐coded system is designed for easy reference:  Findings of the COVID‐US protocol are not recommended for interpretation and clinical management outside individual clinical context.
Findings of the COVID‐US protocol are not recommended for interpretation and clinical management outside individual clinical context.
A‐Lines (Figure 2): A‐Lines are originating from pleura as a result of reverberation artefact arising from the interface between pleural tissue and air within the lung or within the pleural space. These lines are parallel to the pleural and are distributed at equal intervals from the transducer. Importantly, presence of A‐lines is a normal finding, but also can be found in patients with pneumothorax. Only when ‘lung sliding’ phenomenon is observed due to parietal and visceral pleura movements in relation to each other during respiration, the finding suggests the absence of pneumothorax.
Figure 2.

A‐Lines.

B‐lines (Figures 3 and 4): B‐lines are the artefacts originating from the subpleural space, representing vertical lines from the visceral pleura to the edge of the sector, resembling ‘comet tails’. When interstitial pulmonary changes and accumulation of subpleural water take place, these lines increase in number, and become wider and even confluent. More than 3 or wide or confluent B‐lines are considered significant.
Figure 3.

1 B‐Lines.
Figure 4.

Multiple B‐Lines.

Consolidation (Figure 5): pneumonia.
Figure 5.

Consolidation with bronchogram.

Effusion (Figure 6): Estimate Volume (ml) = 20*Max Depth of effusion (mm).
Figure 6.

Pleural effusion with right lower lobe consolidation.

Figure 7.

IVC.
Figure 8.

M‐mode IVC.
Assessment of IVC can be unreliable as an indicator of fluid status particularly in patients with right heart pathology, pulmonary hypertension, high ventilatory pressures, high intra‐abdominal pressure, morbid obesity, etc.10 Care should be taken in decision‐making in conjunction with clinical picture.

Left heart function (Figures 9 and 10): apical 4‐ and 5‐chamber view
Figure 9.

Apical 5‐chamber view.
Figure 10.

LVOT VTI measurement.
Eyeball estimate left ventricular function from 4‐chamber view. Quantify with LVOT VTI measurement from 5‐chamber view:
It is extremely important that decision shall not be made on a single parameter and need to be carefully interpreted in conjunction with all clinical signs. Ultrasound findings are only used to support decisions made within the usual work‐frame.

Right heart function (Figure 11): apical 4‐chamber view
Figure 11.

TAPSE.
Visually estimate right ventricular function. Quantify with TAPSE measurement from 4‐chamber view or RV‐centric apical view:

Discussion
Lung ultrasound findings of COVID‐19 are non‐specific and include thickening of the pleural line with pleural line irregularity; presence of B‐lines in a variety of patterns (including focal, multifocal and confluent); and presence of consolidation in a variety of patterns (including multifocal small, non‐translobar and translobar with occasional mobile air bronchograms). Pleural effusions are uncommon.2 While chest X‐ray may be relatively insensitive to early lung pathology,11 performing CT chests presents major logistic challenges due to the cardiorespiratory instability and difficulties to ensure adequate infection control and safety for the staff and other patients. Indeed, early reports from limited series suggest that lung ultrasound may perform similarly to the CT chest in the detection of COVID‐19 pulmonary involvement.12 Early identification and isolation of suspicious cases reduce the risk of staff exposure. Comparative imaging acquired on the same day of the same COVID‐19 positive patient illustrates multimodality findings (Figures 12, 13, 14):
Figure 12.
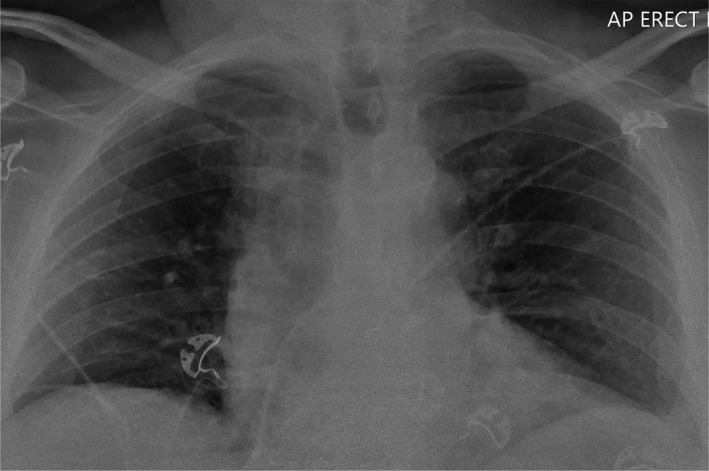
Normal‐looking CXR.
Figure 13.

Lung ultrasound demonstrated pleural thickening of the left lower lobe.
Figure 14.
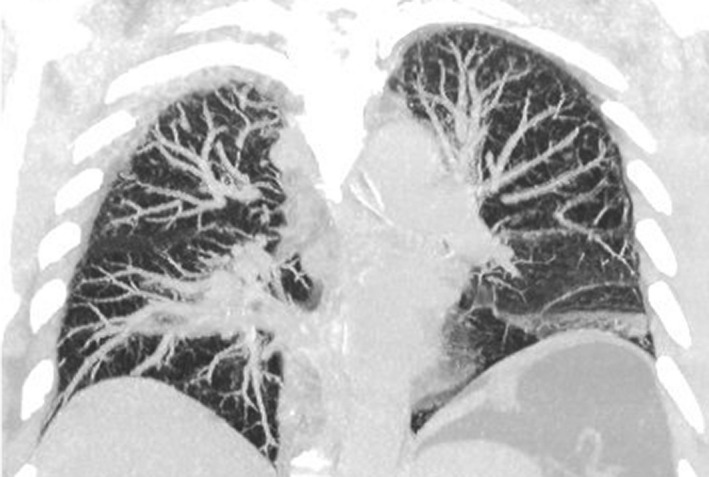
Chest CT demonstrated mild patchy consolidation of the left lower lobe.
Cardiac ultrasonography allows rapid identification and grading of right and left ventricular dysfunction. High‐level of PEEP (above 15 cm H2O) is frequently employed to correct hypoxia in COVID‐19 patients13 and may produce right ventricular (RV) strain and lead to further hemodynamic instability. We suggest that serial monitoring of right ventricular function using TAPSE might be used to detect deteriorating RV function. While many quantification techniques have been proposed and used in clinical and research setting, we feel that TAPSE offers most robust, simple and rapid quantitative choice in the setting of mass ICU admissions.
Given the reported prevalence of myocardial dysfunction in COVID‐19 patients, we suggest serial measurements of LVOT VTI, particularly in patients with progressive haemodynamic instability requiring increasing doses of vasopressors and/or inotropes, or with signs of shock and impaired end‐organ perfusion. Significant or progressive decrease in LVOT VTI value suggests cardiac dysfunction and should prompt more detailed investigations and change in management. Accurate measurement of LVOT VTI is highly angle dependent and should be performed by appropriately trained clinicians to mitigate misdiagnoses. Caution in interpretation is required in patients with arrhythmias and on mechanical ventilation. Other parameters such as MAPSE,14 invasive measurements of LV function could also be considered if LVOT VTI is not measurable or uncertain.
We suggest that major abnormal findings shall be considered for more complementary imaging, such as CT chest for the lung or full echocardiography for the heart. However due to the risk of transporting infected patients and the prolonged staff's exposure, it is a clinical decision to weigh out the risks and benefits individually for such further testing.
In the end, performing ultrasound itself in patients with COVID‐19 carries a risk of cross‐infection. Staff must strictly adhere to the local protocols for personal protective equipment (PPE) as well as cleaning and disinfection of the probe and ultrasound machine between studies.15
Limitation
There are significant limitations for the suggested protocol.
Pulmonary ultrasound has been limited to four points to speed up the assessment, which can predispose to the false‐negative findings for regional disease.
As echocardiographic assessment is centred around ventricular function, there is a chance of missing significant valvular and ischaemic cardiac pathology.
The protocol does not address potential lack of training or recency of ultrasound practice of the operators.
Due to the lack of scientific evidence specific for the COVID‐19 patients, the protocol relies on common sense, extrapolation of other pathology and expert opinion.
Summary
COVID‐US is a simplified bedside cardiopulmonary ultrasound protocol to identify gross cardiopulmonary dysfunction in COVID‐19 patients in a mass surge crisis. A comprehensive examination, and expert guidance, is always recommended when resources allow. Further to this, patient's management should never be based exclusively on image finding but should embrace the entire clinical picture and the most up to date evidence. Future research based on this protocol is considered. Whether such information can lead to interventions that alter the patient's outcome remains to be seen.
Authorship statement
I, Dr. Yang Yang, am submitting this manuscript on behalf of myself and my co‐authors. I confirm that the manuscript has been submitted solely to this journal and is not published, in press, or submitted elsewhere. I confirm that you have prepared your paper and files in accordance with the journal's style and format requirements.
Funding
No funding information is provided.
Conflict of interest
No Funding disclosure or conflict of interest.
Author Contributions
Yang Yang: Conceptualization (lead); Project administration (lead); Resources (lead); Supervision (equal); Visualization (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Konstantin Yastrebov: Conceptualization (supporting); Resources (supporting); Supervision (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (lead). James Anstey: Conceptualization (supporting); Resources (supporting); Supervision (supporting); Visualization (supporting); Writing‐review & editing (lead). Vinodh Bhagyalakshmi Nanjayya: Conceptualization (supporting); Writing‐review & editing (supporting). Sam Orde: Conceptualization (supporting); Resources (supporting); Writing‐review & editing (supporting). Marek Nalos: Writing‐review & editing (supporting). Cartan Costello: Writing‐review & editing (supporting). Nicholas Patrick George Ryan: Conceptualization (equal); Project administration (supporting); Resources (supporting); Writing‐review & editing (supporting).
Acknowledgements
We would like to acknowledgement Prof Andrew Hilton and Dr John Evans of the College of Intensive Care Ultrasound Special interest Group Advisory Committee.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229): 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study G . Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020. 10.1007/s00134-020-05996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323(11): 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein DA. Lung ultrasound in the critically ill. Ann. Intensive Care 2014; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein D. FALLS‐protocol: lung ultrasound in hemodynamic assessment of shock. Heart Lung Vessels 2013; 5(3): 142–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Rudas MOS, Yang Y, Nalos M. Reporting focused lung ultrasound studies in critical care. Recommendations from the College of Intensive Care Medicine Ultrasound Special Interest Group. AJUM 2019; 22(3): 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology 2020. 10.1148/radiol.2020200847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32(1): 1–64. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Ding X, Zhang H, Chen H, Su L, Liu D. Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care 2016; 20: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yastrebov K, Aneman A, Schulz L, Hamp T, McCanny P, Parkin G, et al. Comparison of echocardiographic and invasive measures of volaemia and cardiac performance in critically ill patients. Nature Sci Rep 2020; 10: 4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danish M, Agarwal A, Goyal P, Gupta D, Lal H, Prasad R, et al. Diagnostic performance of 6‐point lung ultrasound in ICU patients: a comparison with chest X‐Ray and CT thorax. Turk J Anaesthesiol Reanim 2019; 47(4): 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Wang S, Liu Y, Zhang Y, Zheng C, Zheng Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19). SSRN Electronic J. 2020. 10.21203/rs.2.24369/v1 [DOI] [Google Scholar]
- 13.Qiu HB, Li XY, Du B, Kang HYJ, Wang YS, Wang F, et al. The keypoints in treatment of the critical novel coronavirus pneumonia patient. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43: E022. [DOI] [PubMed] [Google Scholar]
- 14.Shah A, Nanjayya V, Ihle J. Mitral Annular Plane Systolic Excursion as a predictor of Left Ventricular Ejection Fraction in mechanically ventilated patients. Australasian J. Ultrasound Med. 2019; 22(2): 138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basseal JWS, Juraja M, et al. Guidelines for reprocessing ultrasound transducers. Australas J Ultrasound Med 2017; 20(1): 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


