Abstract
A 46-year-old woman presented to the emergency department with 2-day fever and cough at seven days after returning from Macau. COVID-19 and pneumonia was diagnosed based on the positive real-time RT-PCR tests for oropharyngeal swab samples and the presence of anti-SARS-COV-2 IgG starting from the illness day 11 and post-exposure 18–21 days.
Keywords: Novel coronavirus, SARS-CoV-2, Pneumonia, COVID-19, Macau, Taiwan, Serology, IgG
Introduction
Since first reported in Wuhan, China, in late December 2019, the outbreak of the novel coronavirus now known as SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has spread globally.1, 2, 3, 4 Serological dynamics of the infected persons remain scarce up to now. We report a case of coronavirus disease 2019 (COVID-19) after a travel return from Macau presenting as community-acquired pneumonia with the presence of SARS-CoV-2 IgG antibody at the second week of illness.
Case report
On February 5, 2020, a 46-year-old woman with underlying hypertension presented to the emergency department of a hospital in Tainan, Taiwan, with 2-day fever and cough referred from the outpatient clinic, 7 days after returning from Macau with a 4-day tour (January 21–24, 2020) with her husband and three children. The first confirmed case of the COVID-19 in Macau was announced on January 22, 2020. Given this travel history and progressive airway symptom, she was admitted to the isolation ward with appropriate personal protective equipment.
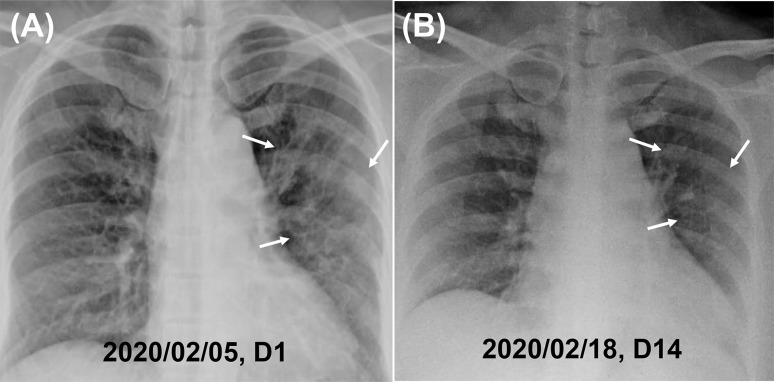
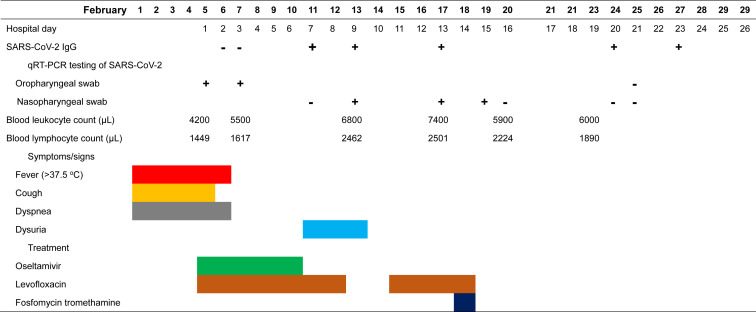
On examination, vital signs were within normal ranges, ear temperature 38 °C, oxygen saturation 97% on room air, and respiratory rate 18 breaths per minute. However, she complicated of dyspnea on exertion. Laboratory investigations showed white blood cell count 4200 per μL (normal 3400–9500 per μL), platelet count 233,000 per μL (143,000–349,000 per μL), hemoglobin concentration 134 g/L (133–172 g/L), serum creatinine concentration 52 μmol/L (70–120 μmol/L), alanine aminotransferase 30 IU/L (<50 IU/L), and lactate 222 U/L (135–225 U/L). A chest radiograph showed patchy densities in the left upper and middle lung fields (Fig. 1 A). The patient received empiric oseltamivir and levofloxacin therapy for pneumonia. Throat swabs were negative for influenza virus A and B using polymerase chain reaction (PCR) method. SARS-CoV-2 was detected in a throat swab sample by real-time reverse-transcription PCR (RT-PCR) for E/RdRp1/RdRp2/N genes (cycle threshold value, 26.34) on hospital day 1 (February 5, 2020). A follow-up chest film at hospital day 14 (illness day 18) showed resolving patchy infiltrates (Fig. 1B). The chest computed tomography at hospital day 26 showed no lesion at left upper lobe. Fever, cough, and dyspnea improved on hospital day 2 (Table 1 ). With three consecutive negative results of real-time RT-PCR tests for SARS-CoV-2 in nasopharyngeal swab, she was discharged after 24 days of hospitalization. None of four members in the family had SARS-CoV-2 detected in their nasopharyngeal swabs sampled after 14 days’ quarantine.
Figure 1.
Chest X-ray films of the case of COVID-19. (A) Hospital day 1: increased pulmonary infiltrations, esp. in left lung field (white arrows). (B) Hospital day 14: Resolution of pulmonary infiltrates at left lung field (white arrows).
Table 1.
Clinical course, laboratory findings, and antimicrobial treatment of the case of COVID-19.
SARS-CoV-2 serology
SARS-CoV-2 (ALLTEST 2019-nCoV IgG/IgM Rapid Test Cassette, Hangzhou ALLTEST Biotech Co., Ltd. Hangzhou, China)5 was utilized for in vitro diagnosis and used to examine the presence of SARS-CoV-2 IgG antibody in seven serum samples (obtained on the hospital day 2, 3, 7, 9, 13, 20, and 23) from the patient. The SARS-CoV-2 IgG antibody was detected in five serum samples since the hospital day 7 (illness day 11, Table 1).
Discussion
Now, the popular diagnostic confirmatory test for COVID-19 is the real-time RT-PCR. In contrast, the chronological dynamics of blood or serum antibodies in the cases of COVID-19 were not well evaluated. In China, a rapid lateral flow immunoassay using a recombinant antigen which is the receptor binding domain of SARS-CoV-2 spike protein to detect both IgM and IgG antibodies, was tested on 397 confirmed cases of COVID-19 and 128 control cases, and showed a promising testing sensitivity of 88.7% and specificity of 90.3%, when all of IgM and IgG positivity, only IgM positivity, and only IgG positivity, were regarded as positive testing results.5 In comparison with real-time RT-PCR, the relative sensitivity, specificity, and accuracy rate of IgG detected by the kit we used, the ALLTEST 2019-nCoV IgG/IgM Rapid Test Cassette, was claimed to be 100%, 98.0% and 98.6%, respectively.6 However, this kit has been tested on serum samples from only 20 RT-PCR confirmed patients and 50 RT-PCR excluded patients and the relative sensitivity for anti-SARS-CoV-2 IgM antibody was 85% (95% CI, 62.1%–96.8%).6 Herein, only the testing results of anti-SARS-CoV-2 IgG antibody was shown (Table 1).
In conclusion, we reported that an initially healthy woman with COVID-19 which developed after Macau travel recovered without evident sequelae, in spite of chest radiographic abnormalities. With an evident travel exposure in this case, the incubation time could be reasonably estimated to be a period of 8–11 days and anti-SARS-COV-2 IgG appeared at post-exposure 18–21 days or the illness day 11. Of course, more serological data should be collected to elucidate the clinical and epidemiological utility of IgM/IgG measurements to detect symptomatic and asymptomatic cases of COVID-19.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Feb 17:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W.H., Teng L.C., Yeh T.K., Chen Y.J., Lo W.J., Wu M.J. 2019 Novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020;53:481–484. doi: 10.1016/j.jmii.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 Feb 27 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Package Insert . Hangzhou ALLTEST Biotech Co., Ltd.; Hangzhou, China: 2020. ALLTEST 2019-nCoV IgG/IgM rapid test cassette. [Google Scholar]