Abstract
Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2), which causes coronavirus disease 2019 (COVID‐19), is highly contagious with devastating impacts for healthcare systems worldwide. Medical staff are at high risk of viral contamination and it is imperative to know what personal protective equipment (PPE) is appropriate for each situation. Furthermore, elective clinics and operations have been reduced in order to mobilize manpower to the acute specialties combating the outbreak; appropriate differentiation between patients who require immediate care and those who can receive telephone consultation or whose treatment might viably be postponed is therefore crucial. Italy was 1 of the earliest and hardest‐hit European countries and therefore the Italian Skull Base Society board has promulgated specific recommendations based on consensus best practices and the literature, where available. Only urgent surgical operations are recommended and all patients should be tested at least twice (on days 4 and 2 prior to surgery). For positive patients, procedures should be postponed until after swab test negativization. If the procedure is vital to the survival of the patient, filtering facepiece 3 (FFP3) and/or powered air purifying respirator (PAPR) devices, goggles, full‐face visor, double gloves, water‐resistant gowns, and protective caps are mandatory. For negative patients, use of at least an FFP2 mask is recommended. In all cases the use of drills, which promote the aerosolization of potentially infected mucous particles, should be avoided. Given the potential neurotropism of SARS‐CoV‐2, dura handling should be minimized. It is only through widely‐agreed protocols and teamwork that we will be able to deal with the evolving and complex implications of this new pandemic.
Keywords: endoscopic sinus surgery, endoscopic skull base surgery, intracranial dura, olfaction, paranasal sinuses, sinus surgery, skull base
The rapid spread of the coronavirus epidemic has forced epochal changes in our daily habits as well as revolutionizing our usual clinical and surgical practice. Since March 12, 2020, when the World Health Organization (WHO) officially announced that the coronavirus disease 2019 (COVID‐19) infection represented a real global pandemic, healthcare workers have been implementing strategies to contain the infection while protecting their patients’ and their own health as much as possible. 1 We are also observing heavy restrictions on healthcare resources, which must be redirected to support the management of the pandemic and therefore removed from normal elective clinical and surgical activities.
In common with many international scientific societies, the Italian Skull Base Society wants to offer clinical and behavioral recommendations to adequately deal with the emergency. Our recommendations are in line with the scientific evidence available to date as well as the concrete experience “in the field” of those who are dealing with the epidemic in the hot areas of the infection.
Olfactory and taste loss
In addition to fever, coughing, wheezing, and diarrhea, there are other symptoms that can be helpful in identifying at‐risk patients. Of these, we would emphasize the importance of hyposmia and dysgeusia, which, as confirmed by much scientific evidence from different countries, are configuring as symptoms associated with severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) infection. 2 Moreover, 1 in 6 patients with recent onset anosmia report this as an isolated symptom (isolated sudden‐onset anosmia [ISOA]). 3 It is known that postviral etiologies account for 15% to 40% of smell and taste loss cases. 4 It is important to investigate this symptom, even in telephone consultations, in order to determine the presence of infection. This might help identify otherwise asymptomatic carriers of disease and trigger targeted testing. 5
The therapeutic recommendations for patients with sudden onset of olfactory loss are: social isolation in quarantine for at least 14 days, and starting nasal olfactory training as soon as possible. 6 Omega‐3 supplementation might be helpful in recovery after postviral loss because it was found to be protective against olfactory loss during the recovery period after skull‐base surgery. 7 In patients with isolated anosmia, a short course of budesonide nasal irrigations can be prescribed because it has been shown to improve olfactory ability 8 without compromising the local or systemic immune status. 9
Other coadjuvant treatment options proposed in the past, before the COVID‐19 outbreak, although without a high level of evidence, might be used to facilitate the recovery of the taste‐olfactory function: vitamin A–based nasal drops, 10 oral alpha‐lipoic acid, caroverine, minocycline, and ginkgo biloba. 11 However, the use of oral corticosteroids should be avoided because they could impair viral clearance and therefore interfere with the subsequent course of the infection. 12 Because no clear data are yet available to contraindicate the use of topical nasal steroid therapy, ongoing chronic nasal topical steroid therapy should be continued regularly to avoid exacerbation of allergic and sinonasal symptoms, which could mimic the symptoms of coronavirus infection. 3 A recent study seems to indicate that most patients are likely to recover chemosensory function within weeks or months, paralleling resolution of other disease‐related symptoms. 13 However, further studies on olfactory function recovery will be necessary to shed more light on this issue.
Precautionary rules for healthcare workers
This infection spreads through droplets and therefore the more the operator is exposed to the patient's airways (eg, via outpatients, endoscopic diagnostic procedures, surgery), the greater the risk. The viral load of SARS‐CoV‐2 is higher in the nasal cavity than in the throat, regardless of whether the patient is symptomatic or asymptomatic. 14 Once aerosolized, SARS‐CoV‐2 particles may stay in the air for at least 3 hours. Otolaryngologists and neurosurgeons are therefore at high risk. 15 The anecdotal evidence of the first case of COVID‐19 transmission during an endoscopic transsphenoidal pituitary surgery in Wuhan, China, and resulting in cross‐contamination of 14 healthcare workers, emphasizes the high potential for hospital‐acquired viral infection. 16 This has provoked anxiety toward endonasal endoscopic procedures worldwide. However, recently, Huang et al. 17 provided additional information to clarify that event, reporting that the infected healthcare workers were mainly those who were outside the operation room. Therefore, the impact of surgical‐related exposure in promoting the contamination should be balanced with the equally important role of social interaction between healthcare staff and contaminated surfaces in wards and living areas. 17
No matter the potential contamination pathways, to date, hundreds of healthcare workers have been hospitalized for COVID‐19 in China and Europe, and some of them, unfortunately, have died. Therefore, the recommendations in managing patients with unknown COVID‐19 status (eg, outpatient service) are to wear appropriate personal protective equipment (PPE), including disposable filtering facepiece 2 (FFP2)/N95 mask, water‐resistant gown, gloves, goggles, cap, and full‐face visor shield. In addition, for COVID‐19–positive patients, filtering facepiece 3 (FFP3) mask and/or powered air purifying respirators (PAPRs) should be used. 18
COVID‐19 positivity tests
Nasopharyngeal swab tests, based on reverse‐transcriptase–polymerase chain reaction (RT‐PCR) to identify SARSCoV‐2, are currently available, results returning within 24 hours with time variations depending on the institution. Recently, a newer test from Abbott (Abbott Laboratories, Chicago, IL), with results ready within 15 minutes, is being made available to current practice. These tests might be burdened by a non‐negligible rate of false negatives, ranging from 4% to 30%, depending on the expertise and training of those performing the swabs. 19 The execution of 2 nasopharyngeal swabs, distanced by at least 2 to 4 days, is recommended to improve the sensitivity of the method. Moreover, the accuracy of the swab test can be increased if complemented with chest imaging showing signs of infection. It was suggested that chest computer tomography (CT) could be even more sensitive in detecting COVID‐19 than repeated RT‐PCR test. 20
New immune‐essay methods are becoming available for blood determination of specific immunoglobulin M (IgM) and IgG antibodies for SARS‐CoV‐2, which can provide results in about 20 to 40 minutes. 21 The rapid IgM‐IgG combined antibody test provided by PharmACT (Berlin, Germany) scored a IgM sensitivity of 70% during the early stages of infection (first 4‐10 days), which rapidly increases to 92.3% between day 11 and day 24. In the same period of late infection, the IgG component of the test reaches 98.6% of sensitivity. These new methods, in addition to being faster, offer an overall lower rate of false negatives (ranging from 11% to 13%) and probably will be used more frequently in the upcoming months. 22
Additionally, saliva may serve as a potential, noninvasive material for diagnosing COVID‐19, with emerging preliminary evidence that it might be considered as a more accurate material to detect the novel coronavirus than nasopharyngeal swab. 23 Saliva could be self‐collected by the patient spitting into a sterile container, eliminating the exposure of healthcare services, and subsequently analyzed using nucleic acid extraction and RT‐PCR test. Authors from China reported that SARS‐CoV‐2 was detected in saliva specimens obtained from 91.7% of patients with COVID‐19. 24 Other studies on this issue are required to better understand the role of saliva in fast diagnosis of SARS‐CoV‐2 infection.
Outpatient assessments
It is recommended that all elective and non‐urgent outpatient procedures be postponed. 25 Many consultations and evaluations can be done by telephone or video visits. Telemedicine screening can also be very useful in identifying urgent cases requiring rapid medical care, as well as those patients with alarming symptoms, who need to be directed toward COVID‐19 diagnostic and therapeutic investigations. We recommend wearing appropriate protective devices, encouraging patients to use a surgical mask, maintaining interpersonal distancing of >1 meter, frequent handwashing and alcoholic disinfection, and only admitting patients themselves to consultations (with exceptions made in the cases of minors or disabled patients). 1 , 25 In the outpatient setting, use of a barrier such as an intact surgical mask or a modified valved endoscopy of the nose and throat (VENT) mask (according to Workman et al. 26 ), which enables endoscopy, significantly reduces aerosol spread.
Skull‐base surgery
Data emerging from international clinical experiences show that surgical procedures involving the airways or using them as a surgical corridor, as in transnasal skull‐base surgery, must be prudentially considered high‐risk procedures, at least as long as further evidence is not available. 16 , 18 , 26 , 27 Obviously, it is not the endoscopic technique per se that is risky, but the nasal and pharyngeal anatomical site is hazardous because it may act as a reservoir with a high viral load. Therefore, all endoscopic, microscopic and open surgical procedures involving these anatomical regions must be considered at risk; likewise, surgical procedures on the lateral skull‐base, given the previous scientific evidences documenting the presence of respiratory viruses in the mucosa of the middle ear. 1 , 15
Our current recommendations are summarized in Figure 1 and described as follows:
Elective surgical activities and non‐urgent procedures must be completely suspended.
Only urgent surgical operations (severe trauma, bleeding, infections, abscesses) and non‐deferrable surgical interventions (malignant tumors with critical local extension to brain, orbit, and/or with borderline resectability where a considerable waiting time might be fatal for the prognosis quoad vitam et valetudinem) should be performed. Pituitary tumors or skull‐base lesions with rapidly worsening vision should receive treatment. Similarly, acoustic neuromas, meningiomas, and other tumors presenting with hydrocephalus or symptoms of brainstem compression should be managed quickly. Conversely, slow‐growing tumors associated with progressive symptoms should be evaluated on a case by case basis. Finally, radiation therapy or systemic therapy should be considered as an alternative to surgery whenever possible. 28
There is new evidence for the neurotropism of SARS‐CoV‐2, and a transcribriform route of the SARS‐CoV‐2 to the brain has been suggested. The isolation of SARS‐CoV‐2 RNA in the cerebrospinal fluid would be the most conclusive evidence to document the neurovirulence of SARS‐CoV‐2. 29 Therefore, dura handling during skull‐base surgery should be performed with particular caution, especially in endoscopic endonasal and lateral skull‐base approaches. Extradural surgery should be advised whenever feasible, whereas transdural approaches should be reserved only for selected cases that are of unavoidable necessity.
In preoperative setting, whenever feasible, patients scheduled for surgery should be prepared with povidone‐iodine (PVP‐I) solution delivered by nasal irrigations and oral wash in order to decrease the potential viral load. 30
It is mandatory to test for COVID‐19 in all patients who are candidates for surgery (except for emergency procedures), with at least 2 tests, repeated at a distance of 2 to 4 days, in order to minimize the possibility of false negatives. The last test must be performed within 48 hours prior to surgery.
For COVID‐19–positive patients, procedures should be postponed until after swab test negativization, when feasible. If the procedure is strictly necessary for the patient's survival, surgery must be performed in dedicated negative‐pressure operating theatres with a pre‐established allocated run, which should not interfere with the COVID‐19–free areas. All medical and nursing staff in the operating room must wear FFP3 and/or PAPR devices, goggles, full‐face visor, double gloves, water‐resistant gowns, and protective caps, not only for the entire duration of surgery but for the whole of the patient's stay in the operating room. 16
If testing for COVID‐19 is not available (emergency procedures such as trauma, major bleeding, abscesses), follow guidelines as for positive patients.
Surgical procedures in COVID‐19–negative patients: use the highest individual protection standards (at least FFP2 mask), in consideration of the significant number of false negatives from the swab tests currently used. 16
In endoscopic transnasal approaches, the use of high‐speed drills that promote the aerosolization of potentially infected mucous particles (aerosol‐generating surgery) should be avoided, or at least reduced. Osteotomes such as Kerrison and Citelli rongeurs, circular punches, and chisel and hammer should be preferred, where possible, to minimize the bony drilling. On the other hand, cold surgical instruments and shavers/microdebriders seem to have less aerosolization risk. 26 However, although this might be true for larger particles, we still do not know if it is the case for smaller aerosolized particles. In selected cases it would be advisable to consider transcranial surgery instead of endonasal whenever feasible without additional morbidity for the patient. 16
Mastoid drilling creates droplets and aerosolization of particles and should also be considered aerosol generating surgery. It should thus proceed using standard PPE (face shields and N95). 31 , 32 , 33 Moreover, once the mastoid and middle ear are open, topical PVP‐I solution should be applied to reduce the mucosal viral load. 30
The whole operating area must be considered at risk, not only for the duration of surgery but for the entirety of the patient's stay. It is therefore advisable to minimize the number of staff in the operating room. Likewise, observers, fellows, and residents in training must be excluded from the operating sessions during this period to reduce exposures. 1
Adequate advice should be provided to COVID‐19–negative patients who undergo surgery to adhere to proper hygiene‐behavioral rules during the postoperative period to avoid subsequent superinfections: this should include stressing the imperative of accurate and frequent handwashing, wearing a surgical mask and of social distancing (greater than 1 meter).
FIGURE 1.
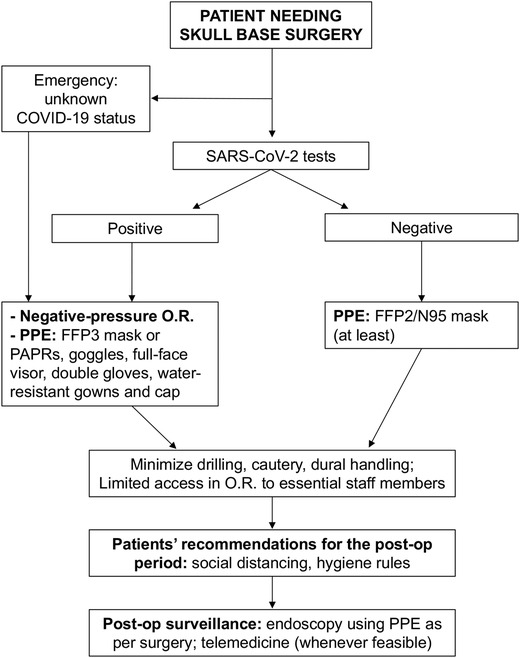
Flowchart for management of patients who are candidates for skull‐base surgery during the COVID‐19 pandemic. COVID‐19 = coronavirus disease 2019; OR = operating room; PAPR = powered air purifying respirator; PPE = personal protective equipment.
Conclusion
These recommendations may require specific patient‐tailored and nation‐based changes according to available facilities and resources as well as on the future evolution of the pandemic.
Acknowledgments
M.T.Z. is a PhD student of the Life Sciences and Biotechnologies course at the Università degli Studi dell'Insubria, Varese, Italy.
How to Cite this Article:Castelnuovo P, Turri‐Zanoni M, Karligkiotis A, et al. Skull‐base surgery during the COVID‐19 pandemic: the Italian Skull Base Society recommendations. Int Forum Allergy Rhinol. 2020;10:963–967.
Potential conflict of interest: None provided.
References
- 1. Kowalski LP, Sanabria A, Ridge JA, et al. COVID‐19 pandemic: effects and evidence‐based recommendations for otolaryngology and head and neck surgery practice. Head Neck. (in press). Epub 09 April 2020. 10.1002/hed.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. (in press). Epub 17 April 2020. 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. Rhinology. (in press). Epub 20 April 2020. 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 4. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 5. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology. (in press). Epub 11 April 2020. 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 6. Soler ZM, Patel ZM, Turner JH, Holbrook EH. A primer on viral‐associated olfactory loss in the era of COVID‐19. Int Forum Allergy Rhinol. (in press). Epub 09 April 2020. 10.1002/alr.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan CH, Rathor A, Krook K, et al. Effect of omega‐3 supplementation in patients with smell dysfunction following endoscopic sellar and parasellar tumor resection: a multicenter prospective randomized controlled trial. Neurosurgery. (in press). Epub 17 January 2020. 10.1093/neuros/nyz559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8:977‐981. [DOI] [PubMed] [Google Scholar]
- 9. Bousquet J, Akdis C, Jutel M, et al. The ARIA‐MASK study group . Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: an ARIA‐EAACI statement. Allergy. (in press). Epub 31 March 2020. 10.1111/all.14302. [DOI] [PubMed] [Google Scholar]
- 10. Hummel T, Whitcroft KL, Rueter G, Haehner A. Intranasal vitamin A is beneficial in post‐infectious olfactory loss. Eur Arch Otorhinolaryngol. 2017;274:2819‐2825. [DOI] [PubMed] [Google Scholar]
- 11. Harless L, Liang J. Pharmacologic treatment for postviral olfactory dysfunction: a systematic review. Int Forum Allergy Rhinol. 2016;6:760‐767. [DOI] [PubMed] [Google Scholar]
- 12. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. (in press). Epub 12 April 2020. 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vukkadala N, Qian ZJ, Holsinger FC, Patel ZM, Rosenthal E. COVID‐19 and the otolaryngologist—preliminary evidence‐based review. Laryngoscope. (in press). Epub 26 March 2020. 10.1002/lary.28672. [DOI] [PubMed] [Google Scholar]
- 16. Patel ZM, Fernandez‐Miranda J, Hwang PH, Nayak JV, Dodd R, Sajjadi H, Jackler RK. Precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Neurosurgery. (in press). Epub 15 April 2020. 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang X, Zhu W, Zhao H, Jiang X. In Reply: Precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Neurosurgery. (in press). Epub 17 April 2020. 10.1093/neuros/nyaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Gerven L, et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID‐19 outbreak. Rhinology. 2020;58:289‐294. [DOI] [PubMed] [Google Scholar]
- 19. Patel ZM. Correspondence: reflections and new developments within the COVID‐19 pandemic. Int Forum Allergy Rhinol. (in press). Epub 15 April 2020. 10.1002/alr.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease (COVID‐19) in China: a report of 1014 Cases. Radiology. (in press). Epub 26 February 2020. 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol. 2020;38:509‐522. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. (in press). Epub 27 February 2020. 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect. (in press). Epub 22 April 2020. 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. To KK, Tsang OT, Yip CC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. (in press). Epub 12 February 2020. 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Bernardi F, Turri‐Zanoni M, Battaglia P, Castelnuovo P. How to reorganize an ENT outpatient service during the COVID‐19 outbreak: report from northern Italy. Laryngoscope. (in press). Epub 05 May 2020. 10.1002/lary.28716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Workman AD, Welling DB, Carter BS, et al. Endonasal instrumentation and aerosolization risk in the era of COVID‐19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. (in press). Epub 03 April 2020. 10.1002/alr.22577. [DOI] [PubMed] [Google Scholar]
- 27. Patel ZM, Fernandez‐Miranda J, Hwang PH, et al. In Reply: Precautions for endoscopic transnasal skull base surgery during the COVID‐19 pandemic. Neurosurgery. (in press). Epub 23 April 2020. 10.1093/neuros/nyaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramakrishna R, Zadeh G, Sheehan JP, Aghi MK. Inpatient and outpatient case prioritization for patients with neuro‐oncologic disease amid the COVID‐19 pandemic: general guidance for neuro‐oncology practitioners from the AANS/CNS Tumor Section and Society for Neuro‐Oncology 2020. J Neurooncol. (in press). Epub 09 April 2020. 10.1007/s11060-020-03488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li YC, Bai WZ, Hashikawa T. Response to Commentary on “The neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients”. J Med Virol. (in press). Epub 04 April 2020. 10.1002/jmv.258244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mady LJ, Kubik MW, Baddour K, Snyderman CH, Rowan NR. Consideration of povidone‐iodine as a public health intervention for COVID‐19: utilization as “personal protective equipment” for frontline providers exposed in high‐risk head and neck and skull base oncology care. Oral Oncol. (in press). Epub 16 April 2020. 10.1016/j.oraloncology.2020.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saadi RA, Bann DV, Patel VA, Goldenberg D, May J, Isildak H. A commentary on safety precautions for otologic surgery during the COVID‐19 pandemic. Otolaryngol Head Neck Surg. (in press). Epub 14 April 2020. 10.1177/0194599820919741. [DOI] [PubMed] [Google Scholar]
- 32. Topsakal V, Van Rompaey V, Kuhweide R, et al. Prioritizing otological surgery during the COVID‐19 pandemic. B‐ENT. (in press). Epub 14 April 2020. 10.5152/B-ENT.2020.20126. [DOI] [Google Scholar]
- 33. Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID‐19 pandemic. JAMA Otolaryngol Head Neck Surg. (in press). Epub 31 March 2020. 10.1001/jamaoto.2020.0780. [DOI] [PubMed] [Google Scholar]


