Abstract
The unprecedented challenges posed by the coronavirus disease 2019 (COVID‐19) pandemic highlight the urgency for applying clinical pharmacology and model‐informed drug development in (i) dosage optimization for COVID‐19 therapies, (ii) approaching therapeutic dilemmas in clinical trial settings, and (iii) maximizing value of information from impacted non–COVID‐19 trials. More than ever, we have a responsibility for adaptive evidence synthesis with a Totality of Evidence mindset in this race against time across biomedical research, clinical practice, drug development, and regulation.
The rapid pandemic spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐associated coronavirus disease 2019 (COVID‐19) is increasingly impacting the healthcare infrastructure, the economic situation, and socio‐cultural interactions globally. Since the onset of SARS‐CoV‐2 spread in late December 2019, more than 900 clinical studies related to COVID‐19 have been registered worldwide as of April 28, 2020, based on a search of clinicaltrials.gov. These include trials investigating existing antiviral and adjunctive immunomodulatory drugs, including large platform clinical trials (e.g., World Health Organization's SOLIDARITY trial and INSERM's Discovery trial). While the urgency of the global threat poses a big challenge to healthcare infrastructure, thousands of ongoing clinical studies aiming to develop new therapies for diseases of high unmet medical need across therapeutic areas are also impacted.
Additionally, there are global public health concerns related to self‐medication with potentially active treatments without a confirmed favorable benefit/risk profile. 1 , 2 This is particularly important in parts of the world where dispensing may not be well controlled, potentially enabling self‐medication in people with advanced age, comorbid health conditions, and polypharmacy. Furthermore, demand for off‐label use of potential COVID‐19 treatments can challenge availability for approved uses (e.g., hydroxychloroquine for systemic lupus erythematosus). 1 Lists of potential COVID‐19 therapies are being maintained by professional organizations. One such resource maintained by the American Society of Health‐System Pharmacists lists over thirty drugs or drug classes, with available preliminary evidence from in vitro, preclinical in vivo, retrospective and prospective clinical evaluations, and dosage in investigational regimens. 3 Such resources highlight gaps in knowledge, conflicting evidence, and heterogeneity in treatment paradigms (e.g., dosage), contributing to overall uncertainty in benefit/risk profile.
Herein, we offer perspectives on the central importance of clinical pharmacology considerations for optimizing investigational COVID‐19 therapy and identify opportunities for model‐informed drug development strategies in addressing some of the unprecedented challenges posed by the pandemic for the broader pharmaceutical research and development community.
Dosage of COVID‐19 Treatments
There is no specific COVID‐19 therapy established yet using any of the available experimental candidate drugs. As the drugs being repurposed “on the fly” for evaluation in COVID‐19 disease either for their antiviral activity or as adjunctive therapies were originally designed and developed for a multitude of other conditions, 1 , 3 defining the optimal dose and duration of treatment will require quantitative clinical pharmacologic considerations. We cannot assume the effective exposures and dosage of lopinavir/ritonavir for HIV, hydroxychloroquine for malaria or systemic lupus erythematosus, anakinra for rheumatoid arthritis, or interferon‐ß‐1a for multiple sclerosis to directly translate to COVID‐19 treatment. Furthermore, extrapolation of available human pharmacokinetic data to critically ill patients (e.g., in acute respiratory distress and associated proinflammatory cytokine release) will need to consider changes in perfusion, plasma protein binding, cytokine‐mediated repression of drug‐metabolizing enzymes, and other unknown factors in these specific populations with complex pathophysiology.
The clinical pharmacology community is rapidly addressing these uncertainties using quantitative translational tools (e.g., physiologically‐based pharmacokinetics, population pharmacokinetics, and mechanism‐based viral dynamics), as exemplified in recent reports aimed at optimizing hydroxychloroquine dosage. 4 , 5 , 6 A recent report of population pharmacokinetics in the critical care setting indicated the variable pharmacokinetic performance of different investigational hydroxychloroquine dosing regimens. 5 Dose selection will also need to consider QT prolongation by this drug and associated proarrhythmic risk. 7 Of note, a QT study or concentration–corrected QT interval analysis for hydroxychloroquine has not been conducted. In a recent integrated modeling exercise, translational pharmacokinetic/pharmacodynamic models for antiviral effect of hydroxychloroquine and pharmacokinetic / corrected QT interval models for chloroquine (assuming a comparable concentration–effect relationship for hydroxychloroquine) were utilized to simulate various dosing regimens. These results identified a need to further evaluate the benefit/risk profile of higher doses (e.g., > 400 mg b.i.d.) of hydroxychloroquine to maximize therapeutic potential. Importantly, this analysis illustrates the value of model‐informed knowledge integration with a Totality of Evidence mindset, while acknowledging and quantitatively considering the impact of data gaps, associated assumptions, and uncertainties. 6
The opportunities for the clinical pharmacology community are plentiful for optimization of COVID‐19 therapeutics, where learning, confirming, and real‐world evidence generation are all compressed in an unprecedented race against time. This applies both for repurposing of drugs approved for other indications and for investigational COVID‐19 therapies sourced from pharmaceutical research and development pipelines based on antiviral potency (e.g., remdesivir) or anticipated benefit as adjunctive therapy based on knowledge of their immuno‐modulatory effects. Quantitative systems pharmacology models of the underlying therapeutic hypotheses can help optimize dosing regimens including design of combinations. Quantitative systems pharmacology models can also interrogate the impact of biological uncertainty underlying clinically important questions such as the risks of continuing treatment with angiotensin‐converting enzyme type 1 inhibitors / angiotensin receptor blockers based on in vitro data demonstrating increased expression of angiotensin‐converting enzyme type 2, which is used by SARS‐Cov‐2 for entry into target cells. 8
Patients in Non–COVID‐19 Clinical Trials who Test Positive for COVID‐19
Although the majority of healthy volunteer clinical trials, except first‐in‐human COVID‐19 vaccine trials, are being paused for subject safety and to decrease pressure on healthcare infrastructure, the situation is not the same for clinical trials in patient populations. The decision to stop treatment with an investigational agent in phase II/III trials in settings where therapy cannot be reasonably interrupted is not trivial. Examples include, but are not limited to, a patient with an aggressive cancer refractory to prior treatments experiencing durable disease control in a clinical trial of an investigational molecularly targeted anticancer agent, or a patient with a life‐threatening rare genetic disease receiving an investigational treatment designed to correct the genetic defect or restore a near‐normal phenotype. If such patients test positive for COVID‐19, they may become candidates for potential treatments for COVID‐19—an unprecedented therapeutic dilemma where initiation of a second technically investigational agent may be necessary. Even if treatment with the investigational agent is paused, depending on its pharmacokinetic half‐life and time course of pharmacodynamic effect, washout of systemic concentrations and/or drug effect may not have occurred at initiation of the COVID‐19 therapeutic. Depending on the stage of development of the non–COVID‐19 investigational agent, clinical pharmacology information on sources of pharmacokinetic variability, clearance mechanisms, and pharmacokinetic and pharmacodynamic drug–drug interaction (DDI) risks (both as a potential object and as a precipitant) may be lacking or limited. Considering the knowledge gaps and uncertainties in our understanding of the clinical pharmacology of potential COVID‐19 treatments, bridging these gaps using the totality of evidence will be necessary. More than ever, in the absence of definitive answers from clinical trials, we will need to adopt a Bayesian mindset and stitch together all available inputs from in vitro, preclinical, and early clinical data, leveraging model‐based methods (e.g., physiologically‐based pharmacokinetics) where feasible, to inform DDI risk assessment. This is not new for clinical pharmacologists engaged in drug development in therapeutic areas like oncology and rare diseases where first‐in‐human studies are routinely initiated in patient populations, requiring a data‐driven principled approach to managing DDI risks. During the present situation, we wish to reinforce the critical importance of clinical pharmacology knowledge integration and readiness for communication of this knowledge at the drug developer–clinical investigator interface, in the context of uncertainty, to maximize the value we can bring for patients.
Role of Clinical Pharmacology in Mitigating Impact of Clinical Trial Disruptions
Despite containment efforts, COVID‐19 has continued to spread, resulting in quarantines and lockdowns handicapping the clinical trial infrastructure worldwide, with regulatory guidelines to support clinical trials during the COVID‐19 pandemic issued by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Missed visits, lost or greatly reduced data collection for pharmacokinetic, pharmacodynamic, safety and efficacy end points, and increased patient dropout are some consequences, challenging trial integrity, data interpretation, and substantiation of evidence. Clinical trialists have responded to these challenges through several innovative maneuvers. These include on‐site visits with careful planning for appropriate distancing/isolation of trial participants to avoid mixing with potentially infected patients, in‐home nurse visits, telemedicine assessments conducted by telephone/video contact rather than on‐site visits, and opportunities for remote source data verification through access to electronic health records when in‐person site visits are not feasible. While case‐by‐case assessments of risk, impact, and mitigation are needed, we identify opportunities from a quantitative clinical pharmacology perspective to maximize value of data, complementing statistical methodologic innovations that have been described to address interruptions in clinical trials. 9 While in‐home visits will result in reduced schedules of pharmacokinetic and pharmacodynamic data collections, the timing of these visits relative to drug administration times can be distributed across visit days and across the trial population to maximize information in the resulting data using principles of optimal sampling, for subsequent population pharmacokinetic‐pharmacodynamic analyses. Thus, a model‐based assessment of pharmacodynamic data may still be feasible to allow “learning” about dose–response and other key objectives of early‐phase drug development while the data may not support the planned inferential statistical analyses. Wearables and digital devices offer additional data collection opportunities for model‐based longitudinal analyses of vital signs and other physiological parameters. 10 Due to the inability to maintain assessments that require site visits (e.g., imaging‐based response assessments) on the protocol‐specified schedule, the actual days of these measurements will be distributed over time. While this may impact primary “landmark” statistical analyses, the resulting data sets with missing and/or unscheduled visits on days different from protocol‐specified days can be amenable to longitudinal disease trajectory modeling. Although statistical power for the protocol‐specified primary analysis may be compromised by a smaller‐than‐intended sample size of the evaluable population, adequately precise estimation of treatment effect may be possible through exposure–response modeling. Similarly, covariate modeling in population analyses can interrogate precision medicine hypotheses even if the number of evaluable patients for the primary statistical analysis in biomarker‐stratified trials is not met. Simulations from such models can support key decisions (e.g., dose selection, patient selection, and proof‐of‐concept), thereby mitigating risk to the broader drug development program, provided overall trial integrity is not otherwise compromised due to the impact of COVID‐19 on efficacy or safety.
Concluding Remarks
The COVID‐19 pandemic has presented unprecedented challenges to public health and drug development. Clinical pharmacologists and model‐informed drug developers are ideally positioned to seize opportunities to enhance therapy optimization, inform complex decisions faced by healthcare providers and clinical trialists, and minimize the impact of disruptions in ongoing clinical trials for non–COVID‐19 drug development using a Totality of Evidence approach (Figure 1). Looking into the future, we hope that the innovations in clinical trial conduct and model‐informed adaptive evidence generation catalyzed by this pandemic will enable long‐term acceleration of Access to Medicines through increased efficiency and shortening of overall drug development timelines. The authors trust that our perspectives on this topic will help reinforce awareness of these opportunities and galvanize a renewed sense of purpose and resilience within the clinical pharmacology community of practice.
Figure 1.
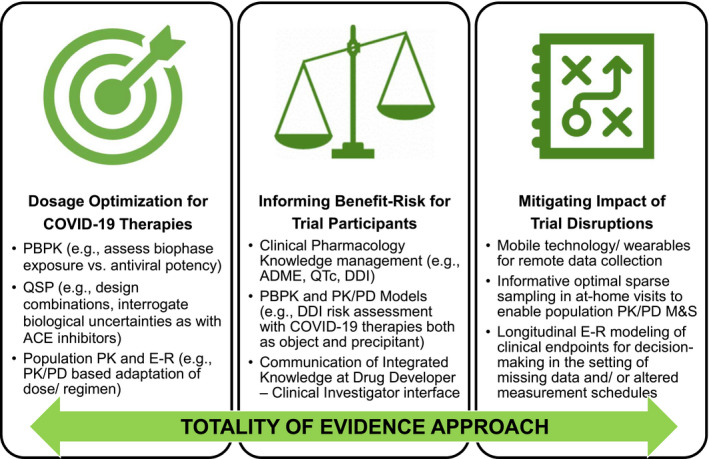
Opportunities for clinical pharmacology and model‐informed drug development during the COVID‐19 pandemic. Applications in ( left panel ) dosage optimization of COVID‐19 therapies, ( middle panel ) informing benefit/risk for clinical trial participants, and ( right panel ) mitigating impact of clinical trial disruptions, with a Totality of Evidence approach. ACE, angiotensin converting enzyme; COVID‐19, coronavirus disease 2019; DDI, drug‐drug interaction; E‐R, exposure‐response; M&S, modeling and simulation; PBPK, physiologically‐based pharmacokinetics; PD, pharmacodynamics; PK, pharmacokinetics; QSP, quantitative systems pharmacology.
Conflict of Interest
K.V., J.Q.D., and L.J.B. are employees of EMD Serono Research & Development Institute, Inc., a business of Merck KGaA, and have declared no competing interests for this work. O.Y. is an employee of Merck Healthcare KGaA, and has declared no competing interests for this work. As an Associate Editor for Clinical Pharmacology & Therapeutics, K.V. was not involved in the review or decision process for this paper.
Funding
No funding was received for this work.
References
- 1. Alpern, J.D. & Gertner, E. Off‐label therapies for COVID‐19 – Are we all in this together? Clin. Pharmacol. Ther. 108, 182–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daily Sabah . Hydroxychloroquine for COVID‐19? Self‐medicating with malaria drugs could be lethal, doctors warn <https://www.dailysabah.com/life/health/hydroxychloroquine-for-covid-19-self-medicating-with-malaria-drugs-could-be-lethal-doctors-warn> (March 29, 2020).
- 3. American Society of Health‐System Pharmacists . Assessment of evidence for COVID‐19‐related treatments <https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table> (2020).
- 4. Yao, X. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin. Infect. Dis. (2020) 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perinel, S. et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID‐19 Patients. Clin. Infect. Dis. (2020) 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia‐Cremades, M. et al. Optimizing hydroxychloroquine dosing for patients with COVID‐19: an integrative modeling approach for effective drug repurposing. Clin. Pharmacol. Ther. 108, 253–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roden, D.M. , Harrington, R.A. , Poppas, A. & Russo, A.M. Considerations for drug interactions on QTc in exploratory COVID‐19 (Coronavirus Disease 2019) treatment. Circulation (2020) 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 8. Sommerstein, R. , Kochen, M.M. , Messerli, F.H. & Gräni, C. Coronavirus disease 2019 (COVID‐19): do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart Assoc. (2020) 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nießl, A. , Beyersmann, J. & Loos, A. Multistate modeling of clinical hold in randomized clinical trials. Pharm. Stat. 19, 262–275 (2020). [DOI] [PubMed] [Google Scholar]
- 10. Izmailova, E.S. , Wagner, J.A. & Perakslis, E.D. Wearable devices in clinical trials: hype and hypothesis. Clin. Pharmacol. Ther. 104, 42–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


