Dear Editor,
We read with interest the article by Morrison et al. on acute hypertriglyceridemia secondary to tocilizumab in patients with severe coronavirus disease (COVID‐19). 1 One patient also had pancreatitis, a dreaded complication of hypertriglyceridemia. Both patients were already on lopinavir/ritonavir before tocilizumab (added day 9 and 13) was added. Propofol was briefly used. We recently had two out of 47 patients with moderate/severe COVID‐19 who were treated with lopinavir/ritonavir and developed significant hypertriglyceridemia resulting in lipemic serum. The first patient (51‐year‐old man) with undiagnosed stage II chronic kidney disease (estimated glomerular filtration rate 61.12 mL/min) and no history of lipid disorder or diabetes mellitus was started on lopinavir/ritonavir (400 and 100 mg twice daily for 14 days) on the 10th day of admission. Blood serum became lipemic on the 10th day of treatment (Figure 1). Serum triglyceride levels ranged between 921.2 and 1071.7 mg/dL (normal range <150 mg/dL). His serum amylase was normal. He was started on bezafibrate and the lipemic serum settled. The second patient (45‐year‐old man) with hypertension, dyslipidemia and uncontrolled diabetes mellitus was started lopinavir/ritonavir monotherapy on the 4th day of admission. His blood was reported to be lipemic on the 11th day of treatment despite being on atorvastatin. Fortunately he completed treatment without any complications. Follow‐up showed no recurrence of lipemic serum in both patients. The second patient had risk factors for hypertriglyceridemia.
Figure 1.
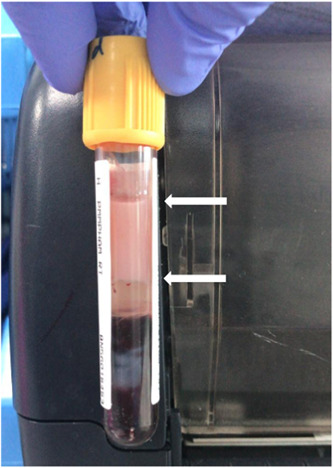
Lipemic serum as shown by a white pinkish top layer (between the arrows)
Lopinavir/ritonavir is known to be associated with lipid abnormality, 2 , 3 more so than tocilizumab. One study showed that at 12 weeks of treatment of lopinavir/ritonavir in patients with human immunodefieciency virus, small but significant increase from baseline in the fasting total cholesterol and triglyceride was observed. 2 Our patients developed lipemic serum within 2 weeks of treatment. In Morrison's cases, it is possible that the lopinavir/ritonavir had contributed to or even caused the hypertriglyceridemia. Therefore it is important for clinicians to be aware and monitor for complications given that the COVID‐19 pandemic will continue and these two medications continue to be used until better treatment options become available.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1. Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR. Letter to the Editor: acute hypertriglyceridemia in patients with COVID‐19 receiving tocilizumab. J Med Virol, 2020. 10.1002/jmv.25907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matoga MM, Hosseinipour MC, Aga E, et al. Hyperlipidaemia in HIV‐infected patients on lopinavir/ritonavir monotherapy in resource‐limited settings. Antivir Ther. 2017;22(3):205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montes ML, Pulido F, Barros C, et al. Lipid disorders in antiretroviral‐naive patients treated with lopinavir/ritonavir‐based HAART: frequency, characterization, and risk factors. J Antimicrob Chemother. 2005;55:800‐804. [DOI] [PubMed] [Google Scholar]


