Summary
The COVID‐19 pandemic has increased the demand for disposable N95 respirators. Re‐usable elastomeric respirators may provide a suitable alternative. Proprietary elastomeric respirator filters may become depleted as demand increases. An alternative may be the virus/bacterial filters used in anaesthesia circuits, if they can be adequately fitted onto the elastomeric respirators. In addition, many re‐usable elastomeric respirators do not filter exhaled breaths. If used for sterile procedures, this would also require modification. We designed a 3D‐printed adaptor that permits elastomeric respirators to interface with anaesthesia circuit filters and created a simple modification to divert exhaled breaths through the filter. We conducted a feasibility study evaluating the performance of our modified elastomeric respirators. A convenience sample of eight volunteers was recruited. Quantitative fit testing, respiratory rate and end‐tidal carbon dioxide were recorded during fit testing exercises and after 1 h of wear. All eight volunteers obtained excellent quantitative fit testing throughout the trial. The mean (SD) end‐tidal carbon dioxide was 4.5 (0.5) kPa and 4.6 (0.4) kPa at baseline and after 1 h of wear (p = 0.148). The mean (SD) respiratory rate was 17 (4) breaths.min−1 and 17 (3) breaths.min−1 at baseline and after 1 h of wear (p = 0.435). Four out of eight subjects self‐reported discomfort; two reported facial pressure, one reported exhalation resistance and one reported transient dizziness on exertion. Re‐usable elastomeric respirators to utilise anaesthesia circuit filters through a 3D‐printed adaptor may be a potential alternative to disposable N95 respirators during the COVID‐19 pandemic.
Keywords: COVID‐19, breathing system filter, anaesthesia circuit filter, respirator, 3D‐printed adaptor
Introduction
The COVID‐19 pandemic has caused a worldwide surge in the consumption of personal protective equipment including disposable N95 or equivalent respirators. These respirators are recommended when performing aerosol generating procedures including tracheal intubation and extubation [1, 2]. For anaesthetists who perform aerosol generating procedures, the critical shortage of these respirators is a pressing issue. National Institute for Occupational Safety and Health (NIOSH)‐approved elastomeric respirators may be suitable alternatives when used with compatible NIOSH or European Certification (CE)‐approved filters such as P100 and P3 [1, 3]. Healthcare personnel can be rapidly fit tested and trained to use them [4]. During the COVID‐19 pandemic, the supply of elastomeric respirator filters may also be depleted. In addition, many re‐usable elastomeric respirators do not filter exhaled breaths [1], but simply have an expiratory one‐way valve. Therefore, elastomeric respirators also require modification when respiratory protection from the user to the patient is also required (e.g. sterile procedures).
High‐efficiency filters routinely used in anaesthesia circuits are designed to filter viruses and bacteria [5]. There are two types of filters; electrostatic and mechanical (also known as pleated hydrophobic). Advantages of electrostatic filters include: low resistance; light weight; small size; and low cost. Disadvantages of electrostatic filters include lower filtration efficiency and vulnerability to liquids (and any microbe contained in the liquid) [5]. Mechanical filters generally have higher filtration efficiency compared with electrostatic filters and are more resistant to liquids. Disadvantages of mechanical filters include higher resistance, heavier weight, larger size and higher cost [6].
Applications of 3D printing in the field of anaesthesia have included: creating airway models for pre‐operative planning [7, 8]; producing airway stents [9, 10]; facial prosthetics [11]; and creating spinal column [12] and bronchial tree [13, 14] education models. Facing the shortage of oxygen delivery devices and personal protective equipment in the COVID‐19 pandemic, 3D printing has been used to convert snorkel masks into CPAP masks for patients [15] and full‐face respirators for healthcare workers [16, 17]. Venturi valves [18] and ventilator splitting devices [19] are also being tested and produced.
In this feasibility study, we designed a 3D‐printed adaptor that allowed 3M™ (St Paul, MN, USA) elastomeric respirators to interface with anaesthesia circuit filters and made a simple modification to the elastomeric respirators to divert exhaled breaths through the filter as well. Fit testing of respirators used during aerosol generating procedures is required. Therefore, it seems reasonable to use both qualitative and quantitative fit testing to evaluate the performance and safety of elastomeric respirators that have undergone the modifications we propose.
Methods
After ethical approval by the Hospital Authority Research Ethics Committee, we recruited eight volunteers who provided their written informed consent. As this was a feasibility study with no comparator group, no power calculation was performed. Primary outcomes were quantitative and qualitative fit testing. Secondary outcomes were end‐tidal carbon dioxide, respiratory rate and volunteer self‐reporting of discomfort.
Due to their widespread use and availability, we chose to design a 3D‐printed adaptor for the 3M 7501 (small) and 3M 6200 (medium) elastomeric respirators. The design of both respirators is comprised of two inhalation limbs on the left and right sides, and an exhalation valve on the front of the respirator. Normally, two filters are placed on the inhalation limbs via bayonet connectors. The 3D‐printed adaptor was designed to fit between the 3M inspiratory limb bayonet connector and a standard 22‐mm outer diameter connector [20] (Fig. 1a). Two of these can be printed for both left and right bayonet connectors, or one side can be closed using a 3D‐printed cap (Fig. 1b). Should the respirator be used for sterile procedures, the expiratory valve can be sealed such that user inhalation and exhalation is via the anaesthesia circuit filter.
Figure 1.
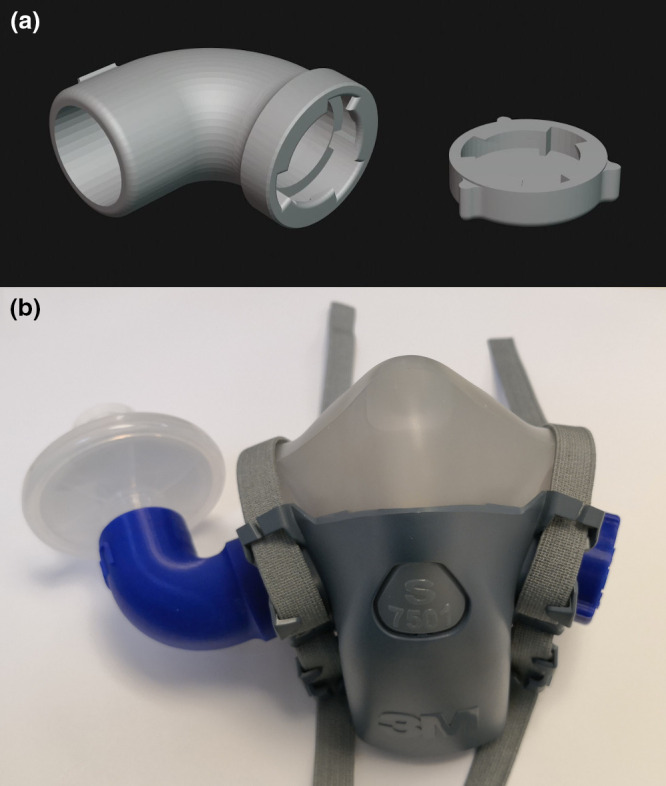
Design of the adaptor and cap. (a) 3D rendering of the 3D‐printed adaptor; (b) Configuration 1: 3D printed and attached to a 3M 7501 respirator and an Undis BVF‐02 anaesthesia circuit filter.
The inspiratory limb adaptor and a cap were designed using SolidWorks software (Dassault Systèmes, Vélizy‐Villacoublay, France) and printed with an Ultimaker S5 3D printer (Utrecht, Netherlands) using polylactic acid (Premium PLA, Formfutura BV, Nijmegen, Netherlands). Printing instructions and a web link to the stereolithography files for the adaptor and cap can be found in online Appendix S1. Polylactic acid was chosen as it is biodegradable, non‐toxic and has a long history of being used for food storage [21] and medical implants [22].
Two configurations were tested. Configuration 1 consisted of inhalation through a single anaesthesia circuit filter and exhalation through the elastomeric respirator exhalation valve (Fig. 1b). Configuration 1 could be used for non‐sterile procedures. Configuration 2 consisted of both inhalation and exhalation through a single anaesthesia circuit filter attached to one inspiratory limb with the exhalation valve occluded. For configuration 2, the inhalation valves were removed and the exhalation valve was occluded by wedging a piece of plastic cut out from a zipper storage bag between the exhalation valve and the head harness assembly (Fig. 2).
Figure 2.
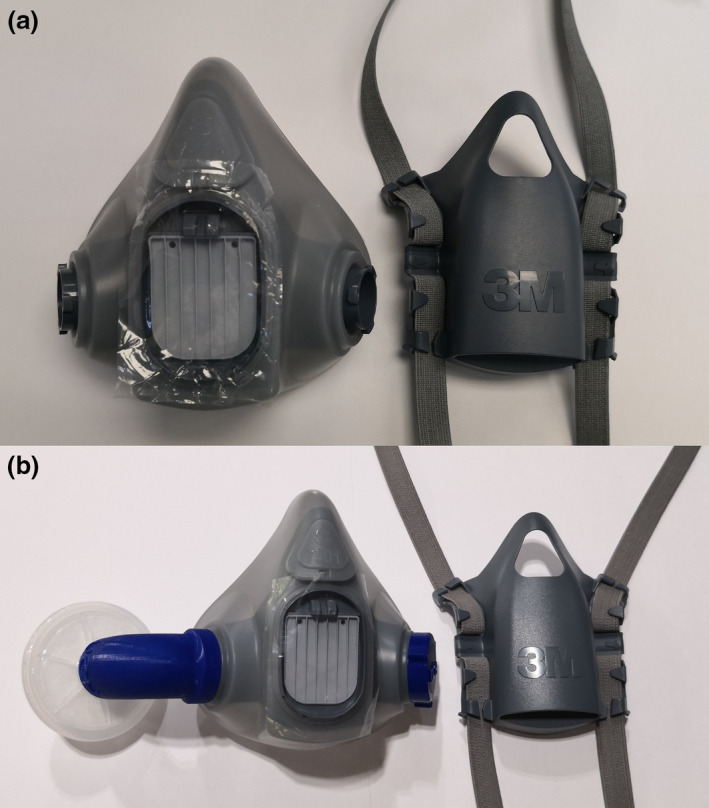
Sealing the exhalation valve. (a) 3M 7501 with a piece of 5 × 7 cm plastic; (b) Configuration 2 with attached adaptor, filter and cap cover (expiratory cover is replaced but is removed for the purpose of clarity).
Qualitative negative and positive pressure leak tests were performed by the eight volunteers. The negative pressure leak test was performed by inhaling with the anaesthesia circuit filter covered by the volunteer’s hand. The positive pressure seal check was performed by exhaling with either the respirator exhalation valve (configuration 1) or anaesthesia circuit filter (configuration 2) covered by the volunteer’s hand. Adequacy of negative and positive qualitative leak tests were self‐reported by the volunteers.
Quantitative fit testing was performed using the PortaCount Pro + 8038 fit tester (TSI Incorporated, Shoreview, MN, USA). The PortaCount N95‐companion mode tests respirators with < 99% filtration efficiency and was used in this feasibility study. The Portacount fit tester measures the concentration of particles 55 nm in size, which carry a negative charge in both ambient air and the respirator. As penetration of these particles through electrostatic filters is insignificant, any 55 nm particles detected inside the respirator must have come from a leak [23]. The fit factor is a ratio of these concentrations. A fit factor in the range of 1 to 200+ is calculated with ≥ 100 constituting a pass [24].
Being the most commonly available anaesthesia circuit filter in our department, we tested the Undis BVF‐02 [25] (Shaoxing, Zhejiang, China) electrostatic filter (without a heat and moisture exchanger). It has a viral and bacterial filtration efficiency of > 99.99%. As most electrostatic filters have a filtration efficiency of < 99% [6], therefore the PortaCount N95‐companion mode was chosen.
Quantitative fit testing can be seen in Figure 3. A 3M 601 fit test adaptor was installed between the elastomeric respirator and the 3D‐printed cap. Initial fit testing was determined as satisfactory if no leaks were detected during pressure seal checks and stable fit factors were obtained during the real‐time display function on the PortaCount Pro + 8038 tester. The real‐time display allows a test subject to experiment with strap tension and other adjustments while watching the effect in real‐time [24]. If the initial tests were unsatisfactory, the volunteers were fitted with an alternate sized respirator and retested.
Figure 3.
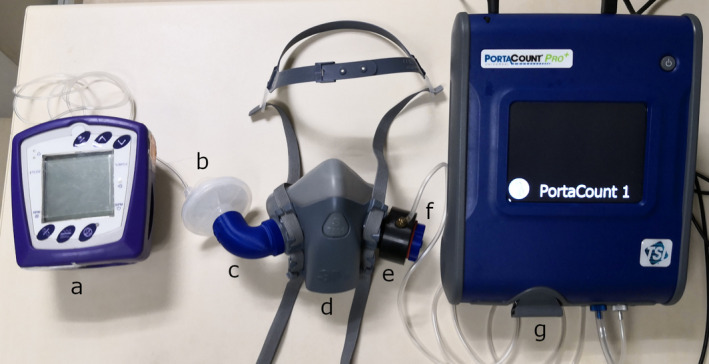
Quantitative fit testing set‐up. (a) Capnocheck‐2 carbon dioxide detector; (b) Undis BVF‐02 anaesthesia circuit filter; (c) 3D‐printed adaptor; (d) 3M 7501 respirator; (e) 3M 601 fit test adaptor; (f) 3D‐printed cap; (g) PortaCount Pro + 8038 fit tester.
Following satisfactory initial fit testing, the volunteers performed full standardised quantitative fit testing [26]. The volunteers performed test exercises consisting including normal breathing; deep breathing; head side to side; head up and down; talking out loud; grimace; and bending with touching toes. Fit testing was performed three times: using configuration 1; using configuration 2; and using configuration 2 after wearing the respirator for 1 h.
End‐tidal carbon dioxide and respiratory rate were measured in configuration 2 at baseline and after 1 h of wear using a Capnocheck‐2 carbon dioxide detector (Smiths Medical, Minneapolis, MN, USA) connected to the anaesthesia circuit filter via its Luer‐lock connector. Volunteers were asked to wear configuration 2 for 1 h as configuration 2 permits inspiration and expiration of tidal volumes only through the anaesthesia circuit filter, potentially requiring a higher work of breathing.
The paired t‐test was used to compare the mean end‐tidal carbon dioxide and respiratory rate. Data were analysed with SPSS 26.0 (IBM Corp, Armonk, NY, USA).
Results
Eight volunteers (five men, three women) completed the study (Table 1). None of the volunteers suffered from cardiorespiratory disease, had a history of smoking, or facial hair or facial abnormalities that may impede fitting of the respirators.
Table 1.
Baseline characteristics of the volunteers.
| No. | Age; y | Sex | Weight; kg | Height; m | BMI; kg.m−2 |
|---|---|---|---|---|---|
| 1 | 38 | F | 55 | 1.58 | 22.0 |
| 2 | 47 | F | 54 | 1.64 | 20.1 |
| 3 | 51 | F | 47 | 1.64 | 17.5 |
| 4 | 34 | M | 67 | 1.67 | 24.0 |
| 5 | 35 | M | 78 | 1.78 | 24.6 |
| 6 | 41 | M | 75 | 1.7 | 26.0 |
| 7 | 49 | M | 68 | 1.71 | 23.3 |
| 8 | 58 | M | 70 | 1.73 | 23.4 |
All volunteers passed qualitative positive and negative pressure leak testing. Four out of five men failed the real‐time quantitative fit testing wearing 3M 6200 (medium) respirators, with the seal becoming unstable during head movement. They were subsequently fitted with 3M 7501 (small) respirators. All three women were fitted with 3M 7501 (small) respirators. All eight volunteers obtained fit factors of 200+ in all three fit tests (range of testing 1 to 200+ with ≥100 constituting a pass) [24].
The mean (SD) end‐tidal carbon dioxide was 4.5 (0.5) kPa and 4.6 (0.4) kPa at baseline and after 1 h of wear respectively (p = 0.148). The mean (SD) respiratory rate was 17 (4) breaths.min−1 and 17 (3) breaths.min−1 at baseline and after 1 h of wear respectively (p = 0.435). Four out of eight subjects self‐reported discomfort; two reported pressure on the face, one reported exhalation resistance and one reported transient dizziness with exertion.
Discussion
This study demonstrated that interfacing an anaesthesia circuit filter with re‐usable elastomeric respirators may be a feasible alternative to disposable N95 respirators for healthcare providers undertaking aerosol generating procedures in patients with COVID‐19 disease. Quantitative fit testing should be mandatory before using re‐usable elastomeric respirators as a significant proportion of volunteers failed the real‐time fit factor test despite having satisfactory qualitative negative and positive pressure seal checks, in line with published guidelines [3, 26]. Once the four volunteers were refitted with a smaller‐sized respirator, quantitative fit testing was excellent for all eight volunteers. Exhalation through the anaesthesia circuit filter was well tolerated with no significant change in end‐tidal carbon dioxide or respiratory rate. Given this is a feasibility study with a convenience sample of only eight volunteers, firm conclusions cannot be drawn.
Elastomeric respirators are designed to undergo repeated disinfection. The cost to print an adaptor and a cap using the recommended polylactic acid as a material is approximately £3 sterling ($3.73 US, 3.5 Euros). We recommend, should these items be required for use, they should be treated as single‐use, disposable items as their integrity and safety after disinfection cannot be guaranteed. Other materials may be suitable for 3D printing the adaptor if they are airtight and biocompatible; however, such use requires communication with regulatory bodies within the country before use as stringent standards must be followed [27, 28, 29, 30, 31].
We sealed the exhalation valve with a piece of plastic in configuration 2 forcing exhalation through the anaesthesia circuit filter. For convenience, we cut a piece of plastic from a zipper storage bag and placed it between the exhalation valve and the head harness assembly (Fig. 2). This may not be a very reliable method for clinical use. Although the elastomeric respirators were well tolerated, four of our volunteers reported some discomfort. Similar complaints of discomfort are often reported when using different types of respiratory protective equipment including surgical masks, disposable N95 respirators, elastomeric respirators and even powered air‐purifying respirators [32, 33, 34]. We found subjectively that wearing these well‐fitted, tightly sealed respirators resulted in the user’s voice being mumbled and lower in volume. This may affect communication, especially when the exhalation valve is sealed. It was possible to understand the user’s speech in a quiet environment; however, in noisy environments such as during resuscitation or trauma, the risk of miscommunication may be increased.
Before resorting to using the anaesthesia circuit filter from one’s hospital, it is important to know its specifications. Many filters have filtration efficiencies of < 95% and are therefore inferior to N95 respirators [6]. Most manufacturers recommend that their filters can be used for up to 24 h, but, due to the accumulation of moisture, resistance will increase with prolonged use. Filters with a heat and moisture exchanger demonstrate a greater increase in resistance compared with those without [6]. Filter specifications have important implications in terms of comfort and safety. Sudden blockage of filters is known to occur [35] and elastomeric respirators may be challenging to doff without adequate training.
When contemplating the clinical use of a modified elastomeric respirator, adherence to appropriate regulatory standards is essential. Regulatory organisations such as the Medicines and Healthcare products Regulatory Agency in the United Kingdom [36], Food and Drug Administration in the United States [37] and Health Canada [38] have released guidance on the manufacturing and usage of personal protective equipment including improvised equipment during the COVID‐19 pandemic. It is important that the safety of healthcare providers be maintained by following the guidelines and protocols from the appropriate regulatory agencies.
Limitations of our study include our small sample size, short duration of respirator usage and that only one model of anaesthesia circuit filter was tested. Other limitations include the highest BMI of our eight volunteers was 26 kg.m−2, with the greatest weight being 78 kg. The use of our modification by larger users requires further investigation to assess resistance through the anaesthesia circuit filter to both inspiration and expiration with increased minute ventilation.
This study was designed to be a feasibility study with descriptive outcome measures and no power analysis was done beforehand. We chose to test the respirators for 1 h as most aerosol generating procedures can be completed within this timeframe.
In conclusion, we have modified a NIOSH‐approved elastomeric respirator not intended for medical use to function as an N95 respirator by interfacing with a standard electrostatic anaesthesia circuit filter with and without occluding the built‐in expiratory valve. If N95 or equivalent respirators and NIOSH‐approved filters become difficult to source, anaesthesia circuit filters when combined with well‐fitted NIOSH‐approved elastomeric respirators using an interposed 3D‐printed connector may be a potential solution.
Supporting information
Appendix S1. Printing instructions and stereolithography files for the adaptor and cap.
Acknowledgements
No external funding or competing interests declared.
References
- 1. CDC . Strategies for optimizing the supply of N95 respirators: COVID‐19. 2020. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/respirators‐strategy/conventional‐capacity‐strategies.html (accessed 26/03/2020).
- 2. Public Health England . COVID‐19 personal protective equipment (PPE). 2020. https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control/covid‐19‐personal‐protective‐equipment‐ppe (accessed 10/04/2020).
- 3. Health and Safety Executive . Respiratory Protective Equipment at Work, 4th edn. London: Health and Safety Executive, 2013. https://www.hse.gov.uk/pubns/books/hsg53.htm (accessed 06/05/2020). [Google Scholar]
- 4. Pompeii LA, Kraft CS, Brownsword EA, et al. Training and fit testing of health care personnel for reusable elastomeric half‐mask respirators compared with disposable N95 respirators. Journal of the American Medical Association 2020; 323: 1849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 1 ‐ history, principles and efficiency. Anaesthesia 2011; 66: 31–9. [DOI] [PubMed] [Google Scholar]
- 6. Medicines and Healthcare products Regulatory Agency . Breathing system filters. An assessment of 104 different breathing system filters. Evaluation. London: Medicines and Healthcare products Regulatory Agency, 2004. [Google Scholar]
- 7. Wilson CA, Arthurs OJ, Black AE, et al. Printed three‐dimensional airway model assists planning of single‐lung ventilation in a small child. British Journal of Anaesthesia 2015; 115: 616–20. [DOI] [PubMed] [Google Scholar]
- 8. Han B, Liu Y, Zhang X, Wang J. Three‐dimensional printing as an aid to airway evaluation after tracheotomy in a patient with laryngeal carcinoma. BMC Anesthesiology 2016; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable Airway splint created with a three‐dimensional printer. New England Journal of Medicine 2013; 368: 2043–5. [DOI] [PubMed] [Google Scholar]
- 10. Cheng GZ, Folch E, Ochoa S, et al. Creating personalized airway stents via 3D printing. American Journal of Respiratory and Critical Care 2015; 191: A3717. [Google Scholar]
- 11. Fan S, Chan A, Au S, et al. Personalised anaesthesia: three‐dimensional printing of facial prosthetic for facial deformity with difficult airway. British Journal of Anaesthesia 2018; 121: 675–8. [DOI] [PubMed] [Google Scholar]
- 12. West SJ, Mari J‐M, Khan A, et al. Development of an ultrasound phantom for spinal injections with 3‐dimensional printing. Regional Anesthesia and Pain Medicine 2014; 39: 429. [DOI] [PubMed] [Google Scholar]
- 13. Bustamante S, Shravan CM. 3D printing for simulation in thoracic anesthesia. Journal of Cardiothoracic and Vascular Anesthesia 2016; 30: e61–e63. [DOI] [PubMed] [Google Scholar]
- 14. Byrne T, Yong SA, Steinfort DP. Development and assessment of a low‐cost 3D‐printed airway model for bronchoscopy simulation training. Journal of Bronchology and Interventional Pulmonology 2016; 23: 251–4. [DOI] [PubMed] [Google Scholar]
- 15. Isinnova . Easy – Covid19 ENG. 2020. https://www.isinnova.it/easy‐covid19‐eng (accessed 19/04/2020).
- 16. The Pneumask Project. https://www.pneumask.org (accessed 19/04/2020).
- 17. Greig PR, Carvalho C, El‐Boghdadly K, Ramessur S. Safety testing improvised COVID‐19 personal protective equipment based on a modified full‐face snorkel mask. Anaesthesia 2020; 75: 970–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleinman Z. Coronavirus: 3D printers save hospital with valves. BBC News 2020. https://www.bbc.com/news/technology‐51911070 (accessed 20/04/2020). [Google Scholar]
- 19. Clarke AL, Stephens AF, Liao S, Byrne TJ, Gregory SD. Coping with COVID‐19: ventilator splitting with differential driving pressures using standard hospital equipment. Anaesthesia 2020; 75: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ISO 5356–1:2015(en), Anaesthetic and respiratory equipment — Conical connectors — Part 1: Cones and sockets. 2015. https://www.iso.org/obp/ui#iso:std:iso:5356:‐1:ed‐4:v1:en (accessed 18/04/2020).
- 21. Conn RE, Kolstad JJ, Borzelleca JF, et al. Safety assessment of polylactide (PLA) for use as a food‐contact polymer. Food and Chemical Toxicology 1995; 33: 273–83. [DOI] [PubMed] [Google Scholar]
- 22. Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. Journal of Applied Microbiology 2019; 127: 1612–26. [DOI] [PubMed] [Google Scholar]
- 23. Han HS, Prell M. Penetration of N95 filtering‐facepiece respirators by charged and charge‐neutralized nanoparticles. 2010. https://tsi.com/getmedia/77041e3a‐3cba‐4dea‐aad0‐6831e88d0792/RFT‐007_Penetration‐of‐N95‐Filtering‐Facepiece‐Respirators‐A4‐web?ext=.pdf (accessed 11/04/2020).
- 24. TSI . PortaCount Pro 8030 and PortaCount Pro+ 8038 Respirator Fit Testers. Operation and Service Manual. Revision P. 2015. https://tsi.com/getmedia/76df3dbb‐6d8d‐4d78‐aa24‐5aff19e889e9/8030_8038_PortaCountPro_Manual_6001868?ext=.pdf (accessed 29/04/2020).
- 25. Shaoxing Undis Medical Technology Co . Lt. BVF‐02. http://www.undis.cn/en/product/241.html (accessed 11/04/2020).
- 26. Occupational Safety and Health Administration . Assigned protection factors for the revised respiratory protection standard. USA: Occupational Safety and Health Administration, U.S. Department of Labor, 2009. [Google Scholar]
- 27. ISO 10993–1:2018(en), Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process. 2018. https://www.iso.org/obp/ui#iso:std:iso:10993:‐1:ed‐5:v2:en (accessed 22/04/2020).
- 28.ISO 18562–1:2017(en), Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 1: Evaluation and testing within a risk management process. 2017. https://www.iso.org/obp/ui#iso:std:iso:18562:‐1:ed‐1:v1:en (accessed 22/04/2020).
- 29. ISO 18562–2:2017(en), Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 2: Tests for emissions of particulate matter. 2017. https://www.iso.org/obp/ui#iso:std:iso:18562:‐2:ed‐1:v1:en (accessed 22/04/2020).
- 30. ISO 18562–3:2017(en), Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 3: Tests for emissions of volatile organic compounds (VOCs). 2017. https://www.iso.org/obp/ui#iso:std:iso:18562:‐3:ed‐1:v1:en (accessed 22/04/2020).
- 31. ISO 18562–4:2017(en), Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 4: Tests for leachables in condensate. 2017. https://www.iso.org/obp/ui#iso:std:iso:18562:‐4:ed‐1:v1:en (accessed 22/04/2020).
- 32. Radonovich LJ, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator tolerance in health care workers. Journal of the American Medical Association 2009; 301: 36–8. [DOI] [PubMed] [Google Scholar]
- 33. Roberge RJ, Coca A, Williams WJ, Powell JB, Palmiero AJ. Reusable elastomeric air‐purifying respirators: Physiologic impact on health care workers. American Journal of Infection Control 2010; 38: 381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rebmann T, Carrico R, Wang J. Physiologic and other effects and compliance with long‐term respirator use among medical intensive care unit nurses. American Journal of Infection Control 2013; 41: 1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 2 – practical use, including problems, and their use with paediatric patients. Anaesthesia 2011; 66: 40–51. [DOI] [PubMed] [Google Scholar]
- 36. Medicines and Healthcare products Regulatory Agency . MHRA Guidance on coronavirus (COVID‐19). https://www.gov.uk/government/collections/mhra‐guidance‐on‐coronavirus‐covid‐19 (accessed 28/04/2020)
- 37. U.S. Food and Drug Administration. Center for Devices and Radiological Health . Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID‐19) Public Health Emergency (Revised). 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/enforcement‐policy‐face‐masks‐and‐respirators‐during‐coronavirus‐disease‐covid‐19‐public‐health (accessed 21/04/2020).
- 38. Health Canada . 3D printing and other manufacturing of personal protective equipment in response to COVID‐19. 2020. https://www.canada.ca/en/health‐canada/services/drugs‐health‐products/medical‐devices/covid‐19‐unconventional‐manufacturing‐personal‐protective‐equipment.html (accessed 21/04/2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Printing instructions and stereolithography files for the adaptor and cap.


