The epidemic
Since first reported in Wuhan, China, in late December 2019, the outbreak of the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally.1, 2, 3 As of March 15, 2020, data from World Health Organization (WHO), more than 153,517 confirmed cases of coronavirus disease 2019 (COVID-19) and 5735 deaths have been reported from 143 countries/territories/areas.3 In China, as of March 15, there were 81,048 confirmed cases of COVID-19 and 3204 deaths.3 On March 11, 2020, the WHO declared COVID-19 a pandemic, pushing the threat beyond the global health emergency it had announced earlier.
Reporting criteria in WHO and centers for Disease Control and prevention of the United States (US CDC)
On January 28, WHO provided a surveillance case definition of COVID-19 and suggested for a laboratory test4: Patients with severe acute respiratory infection (fever, cough, and requiring admission to hospital), AND with no other etiology that fully explains the clinical presentation AND in the 14 days prior to symptom onset, had (1) a history of travel to or residence in the city of Wuhan, Hubei Province, China, or patient is a health care worker who has been working in an environment where severe acute respiratory infections of unknown etiology are being cared for; (2) close contact with a confirmed or probable case of COVID-19, or visiting or working in a live animal market in Wuhan, or worked or attended a health care facility where patients with hospital-associated COVID-19 have been reported. On February 27, the epidemic regions were modified as a history of travel to or residence in a country, area or territory that has reported local transmission of COVID-19 disease and added a clinical criteria of a patient with severe acute respiratory infection requiring hospitalization.5
In the US, CDC offered the first criteria to guide evaluation of patients under investigation for COVID-19 on January 17, 2020.6 Patients who have fever and/or symptoms of lower respiratory illness (e.g., cough, shortness of breath) and in the last 14 days before symptom onset, have a history of travel from Wuhan City, China, OR close contact with a person who is under investigation for COVID-19 while that person was ill or an ill laboratory-confirmed COVID-19 patient should be evaluated. Later, a history of travel expanded from Wuhan city to Hubei Province, and even mainland China for those requiring hospitalization on February 1,7 and to affected geographic regions where sustained community transmission has been identified on February 28.8 With the testing capacity of COVID-19 diagnostic testing allowing clinicians to consider testing for a wider group of symptomatic patients, clinicians were strongly encouraged to test for other causes of respiratory illness (e.g., influenza) in the latest update guidance on March 8.9
Reporting criteria in Taiwan
After the announcement of a cluster of pneumonia of unknown etiology in China, initially reported to the WHO China Country Office, on December 31, 2019, Taiwan Centers for Disease Control (Taiwan CDC) organized and held the first meeting of the Expert Advisory Committee of coronavirus disease-2019 (COVID-19) on January 5, 2020 to prepare to combat COVID-19.10 All the committee members agreed to include COVID-19 as one of the category 5 notifiable diseases, and on January 15, 2020, COVID-19 was announced to be included as such. The first reporting criteria of COVID-19 was drafted on January 7, 2020 under an urgent circumstance.
The reporting criteria of COVID-19 in Taiwan included clinical criteria, epidemiologic criteria and laboratory criteria.11 A patient who has both one clinical criteria and one epidemiologic criteria or has one laboratory criteria fulfills the reporting criteria. Once a patient satisfied the reporting criteria, the patient should be reported to Taiwan CDC within 24 h of suspicion or diagnosis, should be admitted to a single bed room, preferable negative pressure isolation room, in a designated hospital and clinical specimens, including oropharyngeal (or nasopharyngeal) swab, sputum, and serum, should be obtained and sent to the designated CDC-contracted virologic laboratories for the detection of SARA-CoV-19.
According to the laboratory results, a reporting case of COVID-19 can be classified as (1) confirmed case: a case satisfied the laboratory criteria; (2) probable case: a case satisfied clinical criteria, though not confirmed by the laboratory tests, and had intimate contact with a symptomatic confirmed case; (3) excluding case: not satisfying the former two conditions.
For each confirmed case (initially a reporting case under investigation), a CDC investigation team would conduct a detailed investigation by interviewing the case or her/his households to try to explore the potential source of infection, and to identify the contacts with whom the confirmed case had intimate contact during the past two weeks of onset. Each contact, once identified, would be notified by the local Health Bureau to a designated hospital for the laboratory test of SARS-CoV-2.
Evolution of reporting criteria of COVID-19 in Taiwan
The detailed and evolving reporting criteria of COVID-19 is shown in Table 1 . Since announced, the clinical cases of COVID-19 increased day by day, and the clinical manifestations were more and more clearly defined. Thus, the clinical criteria of tachypnea or respiratory difficulty was deleted in second version first and criteria 1 was modified from “fever and acute respiratory infection” to “fever or acute respiratory infection” on January 25. With the ongoing epidemic, for the detection of potential cases due to community spread, the clinical criteria 3 of community-acquired pneumonia (CAP) was added on February 28. In cases of CAP, a patient without a history of traveling to epidemic regions could be regarded as a reporting case after the clinical physician excluded other possible etiologic agent and highly suspected of SARs-CoV-2 as the potential etiology.
Table 1.
Evolving reporting criteria of COVID-19 in Taiwan.
| Date of modification (designation) | Clinical criteria | Epidemic criteria | Laboratory criteria | Reporting criteria |
|---|---|---|---|---|
| Jan. 7, 2020 (A) | Have any one of the followings
|
Traveled to Wuhan, China within 14 days of disease onset | – | Fulfilling both clinical and epidemiologic criteria |
| Jan. 20, 2020 (B) | Have any one of the followings
|
Within 14 days of onset, have any one of the followings
|
Have any one of the followings
|
Have any one of the followings
|
| Jan. 25, 2020 (C) | Have any one of the followings
|
Within 14 days of onset, have any one of the followings
|
Same as above | Have any one of the followings
|
| Jan. 31, 2020 (D) | Same as above | Within 14 days of onset, have any one of the followings
2. & 3. Same as above conditions 2 & 3 |
Same as above | Same as above |
| Feb. 7, 2020 (E) | Same as above | Within 14 days of onset, have any one of the followings
|
Same as above | Same as above |
| Feb. 15, 2020 (F) | Same as above | Henan and Zhejiang provinces included as Level-one epidemic region | Same as above | Same as above |
| Feb. 28, 2020 (G) | Have any one of the followings
|
Within 14 days of onset, have any one of the followings
|
Same as above | Have any one of the followings
|
| March 1, 2020 (H) | Same as above | Iran included as an epidemic region | Same as above | Same as above |
SARS-CoV-2 was identified as the etiologic agent of COVID-19 on January 7 in China and a nucleic acid-based detection method was established and distributed by WHO on January 12. Because the first version of reporting criteria of COVID-19 was drafted and announced on January 7, no laboratory criteria was provided initially. The laboratory criteria of detection of SARS-CoV-2 either by virus culture or nucleic acid-based methods was then added in the second version on January 20 and was not modified since established and announced.
Among the reporting criteria, the most evolving criteria was the epidemiologic criteria. In most countries, the initial epidemiologic criteria was relatively simple, clear-cut and included a history of traveling to China or close contact with a person known to have COVID-19. However, Taiwan is close to, 81 miles off the coast of, mainland China and among the 23 million citizens, 850,000 reside in and 404,000 work in China.10 , 12 , 13 Moreover, 2.71 million visitors from the mainland traveled to Taiwan in 2019.14 The connection between Taiwan and China is tight. In addition, COVID-19 occurred just before the Lunar New Year during which time millions of Taiwanese were expected to return from China for the festivals. If the reporting criteria was established initially as other countries, a huge number of patients would satisfy the reporting criteria of COVID-19, and should be admitted to the designated hospitals for laboratory test of SARS-CoV-2, which would subsequently result in the crisis of medical care loading in all the designated hospitals. Taiwan CDC set up a term of “epidemic regions” and expanded the regions step by step, depending on the ongoing epidemics, from only Wuhan area first, then Hubei province, Guangdong province, Henan and Zhejiang provinces etc. Regions or countries outside mainland China, such as Hong Kong, Macau, Korea, and Italy etc., were added in the list of epidemic regions gradually.
Response and impact of the designated hospitals
With the evolving reporting criteria, the designated hospitals could maintain high quality of medical care under an optimal loading and work well without missing any potential cases of COVID-19. However, several issues were raised. First, while the reporting criteria was modified and evolved from time to time, the clinicians who might encounter the potential cases of COVID-19 should keep an eye on the announcement (mostly the website) of Taiwan CDC to timely catch the changing criteria. Second, not only the clinicians but also the potential cases of COVID-19 were confused in whom fulfilled the reporting criteria and should (could) have a laboratory test. Third, while the order of a laboratory test was restricted to the reporting criteria, the clinician was not allowed to test a suspected patient not fulfilling the criteria until the criteria was expanded to those without a history of travel to epidemic regions or contact with a confirmed case. Subsequently, four cases of CAP were confirmed to be caused by SARS-CoV-2 during hospitalization and one of them (National COVID-19 Case Sequence No. 34) inevitably resulted in the first hospital outbreak of COVID-19 in Taiwan. A clinician's judgement cannot be overlooked. Fourth, while the reporting criteria was expanded, the guidance for caring for the reporting cases under investigation was not modified simultaneously, which subsequently resulted in the exhaustion of personal protection equipment and full occupancy of negative pressure isolation rooms.
COVID-19 in Taiwan
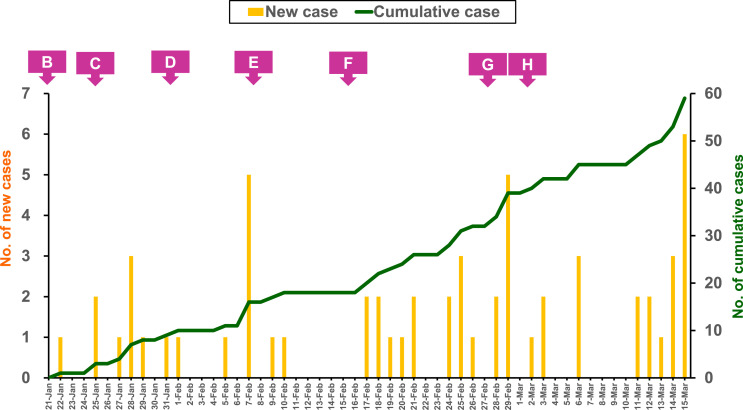
As of March 15, a total of 17,219 cases encountered laboratory tests for SARS-CoV-2; 16.,805 cases were classified as excluding cases and 59 cases as confirmed cases (http://nidss.cdc.gov.tw/ch/SingleDisease.aspx?dc=1&dt=5&disease=19CoV) (Fig. 1 ).15 Of the 59 confirmed cases, 32 cases (54%) were imported and initially mainly from China as expectedly (12 cases, and onset of the last case was on February 2), while later from countries/regions other than mainland China (14 cases unexpectedly from European countries). Twenty-seven cases were domestic in seven clusters. Not surprisingly, six clusters occurred in the households or family members. The remaining cluster occurred in the hospital, which encountered eight cases and included the index case (National COVID-19 Case Sequence No. 34) of unknown source diagnosed 11 days after admission, four health care workers in the ward, two household contacts, one patient and one patient's caretaker in the same ward. Most of the confirmed cases were mild illness or even asymptomatic.16 , 17 Four confirmed cases without travel history encountering a laboratory test due to unexplained pneumonia had a critical disease and one case died eventually.
Fig. 1.
Epicurve of the 59 patients with coronavirus disease 2019 (COVID-19) and the dates (B to H) of modification for reporting criteria in Taiwan from January 21 to March 15, 2020. See Table for the designations of B to H.
Contributor Information
Yhu-Chering Huang, Email: ychuang@adm.cgmh.org.tw.
Ping-Ing Lee, Email: pinging@ntu.edu.tw.
Po-Ren Hsueh, Email: hsporen@ntu.edu.tw.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Feb 17:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-19). Situation Report-55. (https://www.who.int/docs/default-source/coronaviruse/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_2).
- 4.WHO . January 28, 2020. Clinical management of severe acute respiratory infection when Novel coronavirus (2019-nCoV) infection is suspected: interim Guidance. [Google Scholar]
- 5.WHO Global surveillance for COVID-19 disease caused by human infection with the 2019 novel coronavirus. Interim Guid. 27 February 2020 [Google Scholar]
- 6.CDC . January 17, 2020. Update and interim guidance on outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. CDCHAN-00426. [Google Scholar]
- 7.CDC . February 1, 2020. Update and interim guidance on outbreak of coronavirus disease 2019 (COVID-19) CDCHAN-00427. [Google Scholar]
- 8.CDC . February 28, 2020. Update and interim guidance on outbreak of coronavirus disease 2019 (COVID-19) CDCHAN-00428. [Google Scholar]
- 9.CDC . March 8, 2020. Updated guidance on evaluating and testing persons for coronavirus disease 2019 (COVID-19) CDCHAN-00429. [Google Scholar]
- 10.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. J Am Med Assoc. 2020 March 03 doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 11.COVID-19. Taiwan Centers for Disease Control. https://www.cdc.gov.tw/Category/MPage/V6Xe4EItDW3NdGTgC5PtKA.
- 12.Pan T., Yeh J. December 17, 2019. Number of Taiwanese working in China hits 10-year low. Focus Taiwan.https://focustaiwan.tw/business/201912170022 Published. [Google Scholar]
- 13.Statistics on the number of Chinese people working overseas in 2018 [in Chinese] News release. Directorate General of the Budget and Accounting; December 17, 2019. https://www.dgbas.gov.tw/public/Attachment/91217104242H1AK10HM.pdf [Google Scholar]
- 14.Wang S., Lin K. January 6, 2020. Foreign visitors to Taiwan up 7% in 2019. Focus Taiwan.https://focustaiwan.tw/society/202001060014 Published. [Google Scholar]
- 15.COVID-19. Taiwan Centers for Disease Control. Accessed March 16, 2020. https://www.cdc.gov.tw/Disease/SubIndex/N6XvFa1YP9CXYdB0kNSA9A.
- 16.Huang W.H., Teng L.C., Yeh T.K., Chen Y.J., Lo W.J., Wu M.J. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020;53:481–484. doi: 10.1016/j.jmii.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. 2020:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]