Abstract
Coronavirus disease‐2019 (COVID‐19), caused by the highly pathogenic severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), demonstrates high morbidity and mortality caused by development of a severe acute respiratory syndrome connected with extensive pulmonary fibrosis. In this Perspective, we argue that adipocytes and adipocyte‐like cells, such as pulmonary lipofibroblasts, may play an important role in the pathogenic response to SARS‐CoV‐2. Expression of angiotensin‐converting enzyme 2 (the functional receptor for SARS‐CoV) is upregulated in adipocytes of patients with obesity and diabetes, which turns adipose tissue into a potential target and viral reservoir. This may explain why obesity and diabetes are potential comorbidities for COVID‐19 infections. Similar to the recently established adipocyte‐myofibroblast transition, pulmonary lipofibroblasts located in the alveolar interstitium and closely related to classical adipocytes demonstrate the ability to transdifferentiate into myofibroblasts that play an integral part of pulmonary fibrosis. This may significantly increase the severity of the local response to SARS‐CoV‐2 in the lung. To reduce the severity and mortality associated with COVID‐19, we propose to probe for the clinical response to thiazolidinediones, peroxisome proliferator activated receptor γ agonists that are well‐known antidiabetic drugs. Thiazolidinediones are able to stabilize lipofibroblasts in their “inactive” state, preventing the transition to myofibroblasts and thereby reducing the development of pulmonary fibrosis and stimulating its resolution.
Introduction
Coronavirus disease‐2019 (COVID‐2019) caused by the highly pathogenic severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) demonstrates high morbidity and mortality. Progressive consolidation of the lung leading to severe acute respiratory syndrome is recognized as the most common complication in this disease. One of the main reasons for this pulmonary consolidation is an extensive pulmonary fibrosis (PF). PF is likely to be present before the onset of other typical symptoms of a viral infection. General prevalence and incidence of PF increase with age, and PF is especially high in patients over 65 years of age (1). It also has a sexually dimorphic incidence rate, with higher prevalence in males than in females; there is also strong reason to believe that the prevalence of PF in old patients has steadily increased in recent years (1).
PF has been observed in the lungs of patients infected with SARS‐CoV virus (2). This virus shares a high genetic homology with SARS‐CoV‐2. Autopsy of affected cases have demonstrated the appearance of fibrous tissue in alveolar spaces already within the first week of SARS development, with subsequent interstitial and air space fibrosis during the second week and dense septal and alveolar fibrosis during the third week (2), findings further confirmed by computed tomography scans (3). There is broad clinical evidence demonstrating that viral infections are a risk factor for PF (4). Moreover, both viral infections and aging were strongly associated cofactors in PF in this study as well (4). Furthermore, a positive correlation was established between the duration of the illness caused by SARS‐CoV and the degree of interstitial fibrosis (5).
Pathophysiologically, infection with SARS‐CoV induces the expression of transforming growth factor β (TGF‐β) and facilitates its signaling activity. In contrast, infection suppresses the angiotensin‐converting enzyme 2 (ACE2), which is the functional receptor that SARS‐CoV exploits for cell entry. At the same time, ACE2 acts as a negative regulator of pulmonary fibrosis (2). ACE2 is expressed in several different organs, including in the lungs. Under normal conditions, this enzyme is anchored in the plasma membrane of cells. SARS‐CoV binds to ACE2, causes endocytosis, and traffics the virus/ACE complex to endosomes. Interestingly, the affinity between ACE2 and SARS‐CoV‐2 is approximately 10‐ to 20‐fold higher than the affinity between ACE2 and SARS‐CoV (6). Because SARS‐CoV/CoV‐2 both strongly interact with ACE2, the cells expressing ACE are likely to be linked to the progression of PF. In this Perspective, we discuss the role of adipocytes and, more importantly, adipocyte‐like cells in COVID‐19 and describe a possible adjuvant therapy to reduce the severity of this disease.
Role of Adipocyte‐Like Lung Cells in Pathophysiology of PF
The pathophysiology of pulmonary fibrosis is not fully elucidated. However, this condition is known to be a typical component of systemic sclerosis, which is a multisystem fibrotic disorder affecting the skin, lungs, and some other internal organs. In experimental models, systemic sclerosis can be reliably induced by application of bleomycin. This induction is connected with the appearance of biosynthetically highly active myofibroblasts in affected tissues. Myofibroblasts excessively produce extracellular matrix components, hence modifying the lung structure and negatively affecting gas exchange. Whereas the presence of myofibroblasts in PF is well established, it is still a matter of debate where these myofibroblasts originate from. Possible origins for these pulmonary myofibroblasts have been postulated, such as interstitial fibroblasts, pericytes, mesothelial, and epithelial cells (7).
In cutaneous fibrosis, which is also a typical manifestation of systemic sclerosis, the appearance of myofibroblasts is mainly connected with the transdifferentiation of dermal adipocytes located near the interface of the dermis‐subcutis known as adipocyte‐myofibroblast transition (8). Very recently, we formally demonstrated that transdifferentiation of mature adipocytes into myofibroblasts takes place through an intermediate step, dedifferentiation of mature adipocytes into adipocyte‐derived preadipocytes (9), which means that the accumulation of these dedifferentiated adipocytes in affected tissue areas must precede the development of fibrosis (Figure 1, top). In the skin, this process is dependent on the hair follicle cycle, and a causal involvement of TGF‐β is highly likely. Further connecting pulmonary and cutaneous fibrosis is the fact that the TGF‐β/Smad pathway has been found to be critically involved in PF as well (2).
Figure 1.
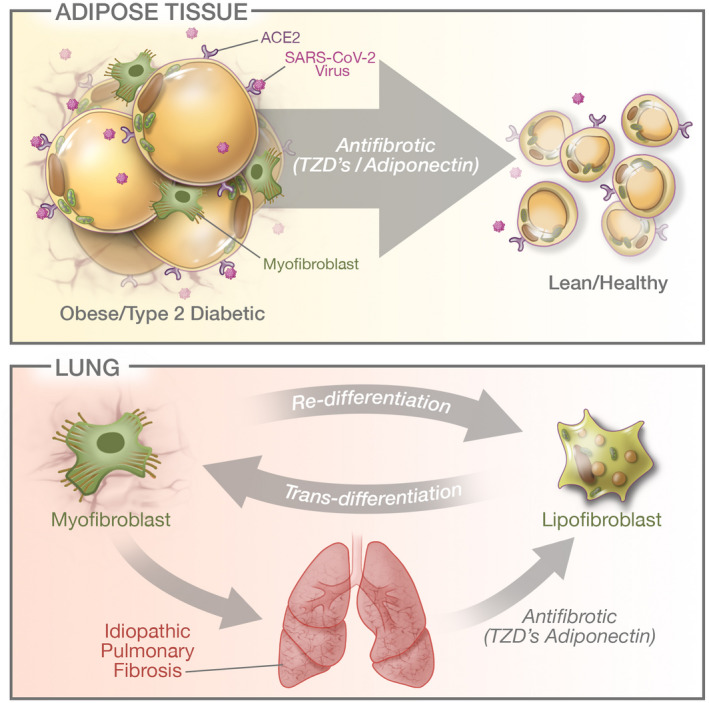
Top: Increased expression of ACE2 (the SARS‐CoV‐2 receptor) on obese and diabetic adipose tissue. This dysfunctional adipose tissue also displays increased fibrosis, at least in part because of a adipocyte‐myofibroblast transition. PPARγ agonists (thiazolidinediones [TZDs]) along with adiponectin (a TZD target as well) are potently antifibrotic and restore functional adipose tissue. Bottom: Lipofibroblasts are the local adipocyte equivalent in the lung and also display the ability to dedifferentiate into myofibroblasts that contribute in an integral way to pulmonary fibrosis. Similar to adipose tissue, TZDs have the potential to act on the myofibroblasts and partially convert them back to lipofibroblasts. In that role, TZDs and adiponectin act as antifibrotic agents as well and have the potential to restore a higher degree of functionality in lung tissue. [Color figure can be viewed at wileyonlinelibrary.com]
Because both cutaneous fibrosis and PF share some important features and because they are both typical components of systemic sclerosis, we propose that they present similar pathophysiological pathways linked to an adipogenic‐myogenic transition. Therefore, we invoke the pulmonary lipofibroblasts (LiF) as the local lung equivalent for PF in a similar fashion that the dermal adipocyte is the source for dermal sclerosis (10).
LiFs carry characteristic lipid droplets and express high levels of perilipin‐2. They are located in the alveolar interstitium adjacent to type 2 alveolar epithelial cells (AEC2) and assist these cells in surfactant production. Approximately 2% of AEC2 cells express ACE2 and therefore represent the biggest pool of ACE2‐expressing cells in the lungs (11). Hence, these cells are the most probable target for SARS‐CoV/CoV‐2. LiFs demonstrate the ability to transdifferentiate into myofibroblasts in vitro in response to hyperoxia and some other factors, and this transition is thought to be the key event in bronchopulmonary dysplasia (12). The appearance of myofibroblasts derived from LiFs was recently confirmed in vivo in mice (13). Whereas the LiF‐myofibroblast transition is typically observed in lung fibroblasts during PF formation, the reverse process, i.e., a myofibroblast‐LiF transition, can be observed during the resolution of fibrosis (13) (Figure 1, bottom). These authors additionally reported a reduction of LiF differentiation markers and a significant increase in fibrotic markers in end‐stage PF. This is consistent with a disappearance of LiFs and their replacement by fibrotic tissue.
LiFs were first established and investigated in neonatal lungs of rodents, whereas their existence in humans was for a long time a topic of controversy (14). Whereas some authors reported the existence of these cells in adult humans, others did not confirm these findings. Only through the use of the neutral lipid fluorescence stain LipidTOX could the existence of resident LiFs in the vicinity of AEC2 in human adult lungs be demonstrated (13). Their established existence in human lungs promotes LiFs from a hypothetically postulated entity to an important player in PF. It is not known whether the pulmonary lipofibroblast is a manifestation of ectopic fat deposition. If so, PF could be a similar pathophysiology as nonalcoholic steatohepatitis; in other words, it could be abnormal fat distribution outside conventional adipose tissue that is the driving force for the lipid deposition in these cells. These are certainly questions that need to be further addressed.
ACE2 is widely expressed in adipocytes and enriched in adipocytes of individuals with obesity and type 2 diabetes. Very little is known about the ACE2 expression in LiFs. Consequently, the question of whether SARS‐CoV/CoV‐2 can directly influence these cells and thereby promote enhanced LiF‐myofibroblast transition should be urgently investigated experimentally. Given their close transcriptional relationship to adipocytes, LiFs are likely candidates for ACE2 expression. If ACE2 is indeed found to be present on LiFs, then these cells may be important targets for the prevention or reduction of COVID‐19–associated PF.
Potential Experimental Approaches to Reduce Severity of COVID‐19
If we assume that SARS‐CoV/CoV‐2 can directly enter the cell and influence the LiF transcriptional program, one way to reduce PF may be the use of PPARγ agonists, which demonstrate potent antifibrotic effects attenuating myofibroblast differentiation and disrupting TGF‐β signaling. Induction of PPARγ leads to an effective reduction of tissue and organ fibrotic disease, including pulmonary fibrosis (15). This effect is at least partially mediated by adiponectin (16). The level of adiponectin in circulation is inversely proportional to adipose mass, is significantly reduced in patients with systemic sclerosis, and is negatively correlated with severity of this disease (17). Circulating levels of adiponectin can be effectively elevated, for example, through application of thiazolidinediones (TZDs), which are PPARγ agonists and established antidiabetic agents (18). Indeed, application of one of these TZDs (rosiglitazone) significantly increased the expression of perilipin‐2 in LiFs, reinforcing the adipogenic phenotype of human lung fibroblasts (13) (Figure 1, bottom). Recently, it was shown that another antidiabetic drug, metformin, accelerated resolution of pulmonary fibrosis by inducing transdifferentiation of myofibroblasts into lipofibroblasts (19). This is also consistent with the finding that metformin decreased the risk of mortality associated with chronic lower respiratory diseases in diabetic patients (16). Whether the application of TZDs and metformin modulates ACE2 expression in adipose tissue and whether TZDs can, through the modulation of ACE2, influence the infectivity of SARS‐CoV should be investigated in future research.
Conclusion
Interaction of both adipocytes themselves and adipose‐like cells with SARS‐CoV/CoV‐2 is involved in pathophysiology of COVID‐19. Adipose tissue can serve as a viral reservoir, whereas transdifferentiation of pulmonary lipofibroblasts into myofibroblasts can contribute to the development of PF and thus is likely to influence the clinical severity of COVID‐19. Application of TZDs and metformin as an adjuvant therapy in COVID‐19 patients may be a worthwhile approach to reduce the development of PF and thus attenuate the severity of the course of disease. This TZD‐based intervention could be started early on upon developing symptoms of COVID‐19. While issues around the long‐term use of TZDs have been raised regarding cardiovascular side effects, these issues have been resolved and deemed nonsignificant. The biggest concern with TZDs is a moderate weight gain; the short‐term use proposed here should not be an issue in this respect either. Patients with type 2 diabetes may benefit a great deal from this approach but would have to be monitored carefully for any signs of hypoglycemia because of enhanced insulin sensitivity.
In addition, because obesity is such a major risk factor for a negative clinical prognosis (20, 21), weight loss per se is a viable strategy as well, even though this is clearly hard to achieve in the absence of any pharmacological interventions.
Funding agencies
PES is supported by NIH grants R01‐DK55758, R01‐DK099110, RC2‐DK118620, P01‐DK088761, and P01‐AG051459. PES is also supported by an unrestricted grant from the Novo Nordisk Research Foundation.
Disclosure
ILK is the managing partner of Wellcomet GmbH. Wellcomet GmbH provided support in the form of salaries for ILK but did not have any additional role in decision to publish or preparation of the manuscript. The commercial affiliation of ILK with Wellcomet GmbH does not alter the adherence to all journal policies on sharing data and materials.
Acknowledgments
We would like to thank Dr. Yu Aaron An, Christy Gliniak, Yingfeng Deng, and Leon Straub for their help with database analysis. We also would like to thank Richard Howdy from VisuallyMedical for the generation of the Figure.
References
- 1. Nalysnyk L, Cid‐Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev 2012;21:355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuo W, Zhao X, Chen YG. SARS coronavirus and lung fibrosis. In: Molecular Biology of the SARS‐Coronavirus. Berlin, Heidelberg: Springer; 2010:247‐258. [Google Scholar]
- 3. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically Ill patients in the seattle region—case series. N Engl J Med 2020. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med 2010;4:759‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tse GM, To KF, Chan PK, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 2004;57:260‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 2020;367:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habiel DM, Hogaboam CM. Heterogeneity of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr Pathobiol Rep 2017;5:101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marangoni RG, Korman BD, Wei J, et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin‐positive intradermal progenitors. Arthritis Rheumatol 2015;67:1062‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Shao M, Hepler C, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 2019;129:5327‐5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kruglikov IL. Interfacial adipose tissue in systemic sclerosis. Curr Rheumat Rep 2017;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Jiang M, Gao L, Huang X. Single cell analysis of ACE2 expression reveals the potential targets for 2019‐nCoV. Preprints 2020. doi: 10.20944/preprints202002.0221.v1 [DOI] [Google Scholar]
- 12. Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal 2014;21:1893‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Agha E, Moiseenko A, Kheirollahi V, et al. Two‐way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 2017;20:261‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahlbrecht K, McGowan SE. In search of the elusive lipofibroblast in human lungs. Am J Physiol Lung Cell Mol Physiol 2014;307:L605‐L608. [DOI] [PubMed] [Google Scholar]
- 15. Kökény G, Calvier L, Legchenko E, Chouvarine P, Mózes MM, Hansmann G. PPARγ is a gatekeeper for extracellular matrix and vascular cell homeostasis: beneficial role in pulmonary hypertension and renal/cardiac/pulmonary fibrosis. Curr Opin Nephrol Hypertens 2020;29:171‐179. [DOI] [PubMed] [Google Scholar]
- 16. Mendy A, Gopal R, Alcorn JF, Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology 2019;24:646‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marangoni RG, Masui Y, Fang F, et al. Adiponectin is an endogenous anti‐fibrotic mediator and therapeutic target. Sci Rep 2017;7:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Straub LG, Scherer PE. Metabolic messengers: adiponectin. Nat Metab 2019;1:334‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kheirollahi V, Wasnick RM, Biasin V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 2019;10:2987. doi: 10.1038/s41467-019-10839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission [published online April 9, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]


