Dear Editor,
The World Health Organization (WHO) has declared that Coronavirus disease 2019 (Covid‐19) is a public health emergency of international concern as it continues to spread worldwide. 1
After a median incubation period of 4 days, fever and cough are the two most common manifestations of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. 2 Physicians worldwide are facing this new disease for which little is known about the full spectrum of its clinical features. For instance, some patients with Covid‐19 associated cutaneous manifestation have been reported, but there is a lack of iconographic and histological documentation. 3 , 4 , 5 , 6 , 7 , 8 , 9 Herein, we describe a febrile rash as the only clinical manifestation of SARS‐CoV‐2 infection in a patient free from pulmonary symptoms.
On March the 7th this year, a 39‐year‐old Caucasian male with no relevant medical history presented to the emergency department with a fever of 39°C, along with a concomitant skin rash that had appeared the same day. This rash was characterized by erythematous and oedematous non‐pruritic annular fixed plaques involving the upper limbs, chest, neck, abdomen and palms, sparing the face and mucous membranes (Fig. 1a–e). Importantly, the patient declared having taken no medication in the days and weeks before the onset of symptoms. His vitals were normal, and he had no signs of upper respiratory tract or pulmonary infection.
Figure 1.
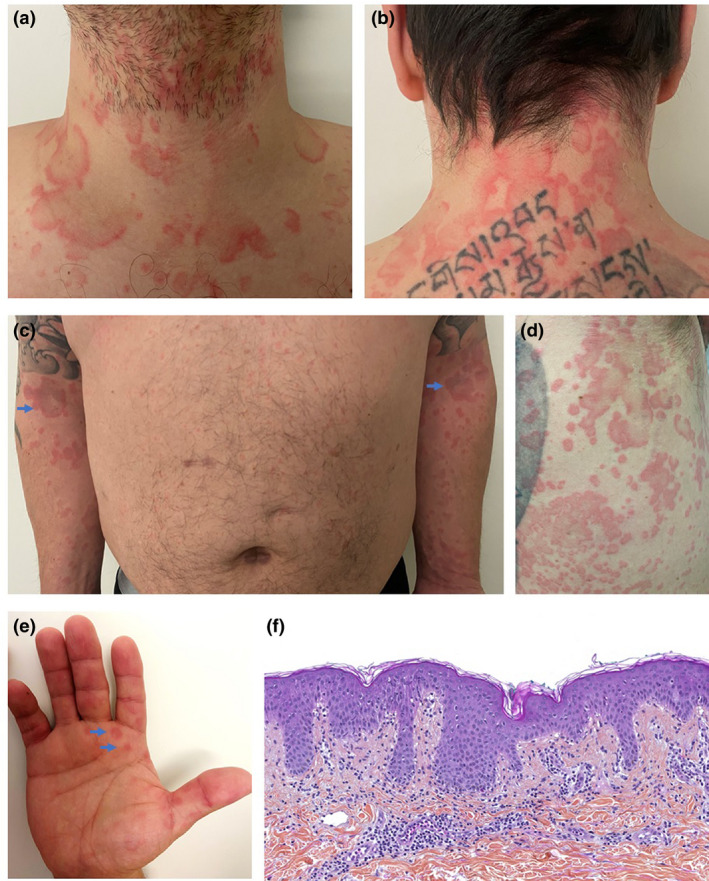
Clinical and histological features of Covid‐19‐associated febrile rash. (a, b) Erythematous, edematous, annular and circinate plaques involving the anterior and posterior neck. (c) Symmetrical distribution of lesions on the upper limbs. (d) Well‐defined polycyclic erythematous plaques of various diameters on the right flank. (e) Annular papules of the palms. (f) HPS ×200. Histological findings were unspecific but consistent with viral exanthemata: superficial perivascular lymphocytic infiltrate, papillary dermal edema, mild spongiosis, lichenoid and vacuolar interface dermatitis, dyskeratotic basilar eratinocytes, occasional neutrophils but no eosinophils within the dermal infiltrate.
The patient promptly reported to the physician that he had been in contact 5 days earlier with a family member, who was afterwards tested positive for SARS‐CoV‐2. Quantitative reverse‐transcriptase–polymerase‐chain‐reaction (qRT‐PCR) assay performed on both nasopharyngeal swab and sputum sample revealed the presence of SARS‐CoV‐2 RNA. The search for other respiratory viruses, such as influenza A and B viruses, rhinovirus, and common coronaviruses was negative, as was the blood culture. Blood count, electrolytes, C‐reactive protein and anti‐DNA antibodies were normal too. The histological examination of the skin showed non‐specific changes, compatible with viral exanthemata: predominantly superficial perivascular infiltrate of lymphocytes without eosinophils, papillary dermal oedema, subtle epidermal spongiosis, mild lymphocyte exocytosis, lichenoid and vacuolar interface dermatitis with occasional dyskeratotic keratinocytes in the basal layer (Fig. 1f). No virally‐induced cytopathic alterations or intranuclear inclusions were present. Direct immunofluorescence was negative.
Despite normal chest radiograph on admission, a chest CT scan showed bilateral and peripheral ground‐glass and consolidative pulmonary opacities, highly suggestive of SARS‐CoV‐2 infection. 10
On March the 8th, the patient started oral hydroxychloroquine sulfate 200 mg three times per day for 10 days with a daily monitoring of SARS‐Cov‐2 qRT‐PCR on nasopharyngeal swab. No pulmonary symptoms developed. On March the 14th, the rash fully recovered and laboratory tests for SARS‐CoV‐2 qRT‐PCR became negative on March the 20th.
Our case report provides two important facts that need highlighting. Firstly, Covid‐19 disease can present with a distinctive rash, which is histologically similar but clinically different to classic viral exanthemata. Indeed, the annular, polycyclic and circinate appearance of the skin lesions differed from classic paraviral rashes in adults, as did the papules on the palms. In addition, unlike viral infection‐associated urticaria, the plaques were both fixed and non‐pruritic. Secondly, a febrile rash may be the only clinical manifestation of Covid‐19. As the outbreak spreads around the world, including in medically underdeveloped countries, choices will have to be made regarding which patients need to be tested or not for SARS‐CoV‐2. We suggest that patients presenting with a febrile rash during the current pandemic should be tested.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of his case details.
Funding source
none.
References
- 1. Team E editorial . Note from the editors: World Health Organization declares novel coronavirus (2019‐nCoV) sixth public health emergency of international concern. Eurosurveillance 2020; 25: 200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020. [Epub ahead of print]. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 4. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for Dengue. J Am Acad Dermatol 2020; 82: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahé A, Birckel E, Krieger S et al. A distinctive skin rash associated with Coronavirus Disease 2019 ? J Eur Acad Dermatol Venereol. 2020. [Epub ahead of print]. 10.1111/jdv.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Estébanez A, Pérez‐Santiago L, Silva E et al. Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venereol 2020. [Epub ahead of print]. 10.1111/jdv.16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su CJ, Lee CH. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol 2020. [Epub ahead of print]. 10.1111/jdv.16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry D, Ackerman M, Sancelme E et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. [Epub ahead of print]. 10.1111/jdv.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mungmungpuntipantip R, Wiwanitkit V. COVID‐19 and cutaneous manifestations. J Eur Acad Dermatol Venereol 2020. [Epub ahead of print]. 10.1111/jdv.16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ai T, Yang Z, Hou H et al. Correlation of Chest CT and RT‐PCR Testing in Coronavirus Disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020. [Epub ahead of print]. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]


