Abstract
The World Health Organization announced the coronavirus disease 2019 (COVID‐19) outbreak a pandemic on 12 March 2020. Although being in proximity to China, the original epicenter of the COVID‐19 outbreak, Taiwan has maintained a low number of COVID‐19 cases despite its close social ties and heavy traffic between Taiwan and China. Containment strategies executed by the Taiwanese government have attracted global attention. Similarly, in‐hospital settings, high alertness and swift responses to the changing outbreak situation are necessary to ensure hospital staff members' safety so they can continue to save patients' lives. Herein, we present infection control measures that can be adopted in hospital settings that were executed in a Taiwanese hospital to confront the COVID‐19 pandemic, including emergency preparedness and responses from the hospital administration, education, surveillance, patient flow arrangement, the partition of hospital zones, and the prevention of a systemic shutdown by using the “divided cabin, divided flow” strategy. The measures implemented by a Taiwan hospital during the COVID‐19 pandemic may not be universally applicable in every hospital. Nonetheless, the presented infection control methods have been practically executed and can be referenced or modified to fit each hospital's unique condition.
Keywords: COVID‐19, infection control, novel coronavirus, SARS‐CoV‐2, Taiwan
1. INTRODUCTION
In December 2019, a novel coronavirus infection emerged in Wuhan, China. 1 This has been the third coronavirus to cause severe respiratory infections in the past 2 decades, following severe acute respiratory syndrome (SARS) coronavirus virus in 2003 and Middle East respiratory syndrome coronavirus in 2012. 2 This emerging infectious disease has already infected more than 250 000 people and caused more than 10 000 deaths globally within 3 months. It has spread to at least 146 countries and territories on six continents. The World Health Organization officially named the disease “COVID‐19” and announced the COVID‐19 outbreak a pandemic on 12 March 2020. Since the COVID‐19 outbreak extended from primarily Asia to the European Union, the United Kingdom, and the United States at the beginning of March, it has posed an enormous challenge on health care and economic systems worldwide.
The clinical manifestations of COVID‐19, which is caused by SARS coronavirus 2 (SARS‐CoV‐2), have three major characteristics that differentiate it from SARS: (a) not all infected people develop fever symptoms; (b) asymptomatic infected cases may transmit the virus, and a familial cluster of COVID‐19 indicated potential person‐to‐person transmission during the incubation period 3 ; and (c) although the mortality rate of COVID‐19 is lower than that of SARS, the efficacy of COVID‐19 to spread is considerably higher. 4
Being in proximity to China, the original epicenter of the COVID‐19 outbreak, Taiwan has maintained a low number of COVID‐19 cases despite its close social ties and heavy traffic between Taiwan and China. Containment strategies executed by the Taiwanese government have attracted global attention. One of the reasons for the vigilant response of the Taiwanese public health authorities with the full cooperation of the health‐care system at all levels comes from the fact that Taiwan lost 81 lives during the SARS pandemic 17 years ago. The first nosocomial SARS outbreak in a hospital infected 150 people and caused 35 deaths. A total of 11 health‐care workers sacrificed their lives combatting the SARS outbreak in 2003. This left a traumatic memory to a whole generation of health‐care workers in Taiwan; therefore, Taiwan maintains a high degree of alertness and thus responds rapidly to SARS‐like emerging infectious diseases.
2. WHY IS KEEPING A HOSPITAL SAFE CRUCIAL DURING AN EPIDEMIC?
To face the threat of an emerging infectious disease epidemic, such as COVID‐19, countries around the world develop strategies such as containment, delaying transmission/outbreak, and mitigation. 5 Highly effective contact tracing and case isolation may control a new outbreak of COVID‐19 within 3 months. 6 Hospitals are key to the success of each strategy because they provide laboratory diagnosis and medical care for patients as well as isolate suspected and confirmed cases to stop viral transmission. In addition, hospitals are regarded as the stronghold of protecting sick people during an epidemic when fear and anxiety increases in the general population. 7
A nosocomial outbreak in a hospital can cause significant morbidities and mortalities among patients and health‐care workers alike. The rapid transmission of the virus among health‐care workers, particularly when accompanied by fatalities in doctors, nurses, and paramedics, can cause enormous fear and panic among hospital staff and the general population. Many reported nosocomial outbreaks of COVID‐19 increased so rapidly that they led to the collapse of whole health‐care systems. The considerably higher mortality rate of COVID‐19 in Wuhan than that in other areas of China 8 may result from the malfunctioning of the health‐care system in Wuhan following the waves of nosocomial transmission and a sudden surge in the disease burden. Because hospitals are on the frontline meeting medical needs and providing a sense of security and confidence to the general population, avoiding nosocomial outbreaks in hospitals during an emerging infectious disease epidemic is essential.
Preserving hospital functions requires effective infection control strategies and measures. Because many hospitals in Taiwan experienced the SARS pandemic in 2003, measures were taken against the incoming COVID‐19 outbreak at the beginning of the epidemic. In the following sections, we summarize emergency response strategies and infection control measures that hospitals can apply under an evolving epidemic. Herein, we report practical and detailed measures adopted by Kaohsiung Medical University Hospital (KMUH), a 1600‐bed tertiary care medical center in southern Taiwan. Many of hospital staff members experienced the SARS outbreak in 2003 and cared for confirmed cases. Annual drills to cope with the potential re‐emergence of SARS have been conducted for 17 years. Although an emergency response plan against emerging infectious diseases is revised annually, timely and practical modifications of the plan were made to confront the COVID‐19 outbreak.
3. INFECTION CONTROL MEASURES OF KMUH
Before forming a reinforcement scheme for hospitals confronting COVID‐19, analyzing potential weak points in all aspects regarding infection control and nosocomial outbreak prevention first is critical. This type of analysis was conducted by the KMUH emergency response task force (Figure 1).
FIGURE 1.
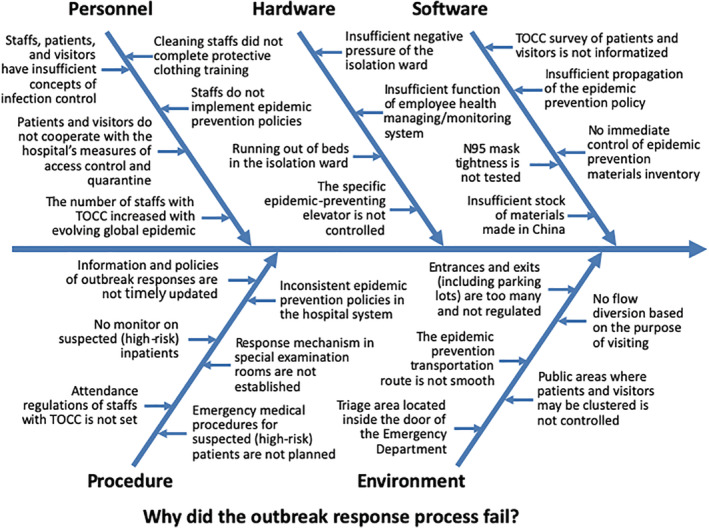
Analysis of the potential weak points of emergency response to the COVID‐19 (coronavirus disease 2019) outbreak
3.1. First stage: Preparedness and education
Before an infected case enters the hospital setting, education for health‐care workers should be implemented and precautionary vigilance should be enhanced for preparedness. After the first imported confirmed case of COVID‐19 in Taiwan was announced by Taiwan's Centers for Disease Control (CDC) on 21 January 2020, our response team was immediately summoned. The “response task force for COVID‐19” comprises the “medical care team,” “nursing team,” “infection control team,” and “administrative team.” Several interventions were promptly implemented with the effort and cooperation of the four teams:
Briefings on COVID‐19 epidemic updates are conducted, and infection control measures are checked daily to integrate the information and enhance interdepartmental cooperation.
Updated information is distributed to staff by using a simplified algorithm to incorporate travel health notices, government quarantine instructions, and the frequently revised COVID‐19 notification definitions from the national public health authorities.
Educational programs are conducted for all health‐care workers, administration staff, clerks/interns, and hospital cleaning staff. Online learning programs are made available.
Storage and the daily consumption of personal protective equipment (PPE) are regularly checked to ensure that adequate stock of safety equipment is available, including major items such as face masks, N95 masks, goggles, protective clothing, surgical masks, and face shields. Powered air‐purifying respirators (PAPRs) used during tracheal intubation are also checked daily.
3.2. Second stage: Surveillance, patient flow arrangement, and partition of hospital zones
3.2.1. Surveillance
To prevent febrile cases with recent international travel history from entering the hospital building and risking consequent nosocomial transmissions, 9 hospital entrance control is required. Most hospital entrances were closed except for two designated gates (Figure 2). We established quarantine stations at the designated entrances, which employ the following measures.
FIGURE 2.

Hospital layout comprising the outdoor clinic section, drive‐through pharmacy, outdoor screening area, and portable X‐ray tent outside the emergency room. Visitors enter the hospital through two designated gates
Fever screening
Fever is a major symptom of COVID‐19; therefore, screening for febrile cases before people enter the hospital is necessary. In KMUH, fever surveillance is conducted using infrared thermal imaging cameras and forehead thermometers. All visitors must pass the thermal imaging camera at hospital gates. Forehead thermometers are used to double‐check people with suspected fever.
Checking for history of travel, occupation, contact, and clusters
Obtaining accurate information of all hospital visitors' international travel history at the beginning of the epidemic is crucial because most COVID‐19 cases in Taiwan were imported. In Taiwan, all citizens use a National Health Insurance (NHI) card to access medical services. Because the immigration and customs database was integrated with the NHI database by the Taiwanese government, hospital staff can confirm the travel history of all visitors by reading their NHI card. KMUH designed mobile working stations that can access NHI cards by modifying nurses' working carts in regular wards (Figure 3). Before entering the hospital building, all visitors, including patients, caregivers, and family members, are asked to wear surgical masks and provide their NHI card to confirm their travel history. Moreover, their full history of travel, occupation, contacts, and clusters (TOCC) is collected in a questionnaire. A handstamp is used for a 1‐day pass certificate to reduce the man power required to recheck body temperatures and travel history (Figure 3). Anyone with a recent travel history to COVID‐19 endemic areas is referred either to the emergency department or to an outdoor clinic for further management depending on the patient's medical needs (described in the following paragraphs).
FIGURE 3.

National Health Insurance working stations to access NHI cards and hand stamps used for 1‐day passes
Hospital staff sign‐in system
Hospital staff members' health status and travel history are closely monitored. The information technology (IT) department of KMUH links staff members' identification (ID) number with the NHI database to construct a staff sign in system. It is compulsory for all staff members to sign in using their staff ID card and have their body temperature checked at the entrance before work to monitor and detect all risks of intrahospital transmission between health‐care workers early.
3.2.2. Patient flow arrangement
During an epidemic, medical facilities or hospitals arranging separate zones for patient care is common: The green zone is the clean area for cases without COVID‐19 symptoms, and the red zone is the area to care for suspected or confirmed COVID‐19 cases. The arrangement of these settings should be individualized according to each hospital's needs and capacity. The distribution of these zones should be dynamically adjusted or expanded for the extent of an outbreak. Then, a traffic control scheme for patient flow can be set up according to the distribution of the zones mainly to prevent the interflow of people between red and green zones. 10
During the initial stage when there were few cases in Taiwan, we arranged the patient flow by the level of risk of COVID‐19 infection and mortality (Table 1). Although this patient flow control design cannot guarantee the absolute separation of potential cases from the uninfected population given the 14‐day incubation period of COVID‐19 and the uncommon but possible transmission from asymptomatic cases, this is a critical and essential method of delaying viral transmission during the containment stage. We assigned the following routes according to different target groups:
TABLE 1.
Allocation according to the risk of COVID‐19 infection
| Allocation | Symptoms of infection: Yes | Symptoms of infection: No |
|---|---|---|
| Risk for COVID‐19 infection a : High | ER | Outdoor clinic section |
| Risk for COVID‐19 infection: Low | The usual outpatient department | Drive‐through pharmacy for prescription refill |
Abbreviation: ER, emergency room
In terms of travel history, contact history, or other clinical criteria provided by Taiwan's Centers for Disease Control.
Patients with fever or respiratory tract symptoms with travel history to COVID‐19 endemic areas: These patients are referred directly to the emergency room (ER) for triage and treatment. Restricting such patients to confined areas reduces potential environmental contamination and personnel exposure to infection.
Patients without symptoms or signs of infectious diseases but have travel history to COVID‐19 endemic areas: For patients who returned from regions with travel alerts and have medical needs other than infectious causes, such as headache, dizziness, or urticaria, we refer them to the “outdoor clinic section” (Figures 2 and 4) for further management.
FIGURE 4.

Outdoor clinic section used to treat patients without symptoms or signs of infectious diseases with travel history to COVID‐19 endemic areas
3. Patients who need to refill their regular prescriptions: In Taiwan, patients with certain chronic conditions with regular follow‐ups, such as diabetes or hypertension, typically refill their prescriptions monthly at hospital pharmacies without seeing their doctors for a 2‐ or 3‐months period. This is known as the “refill prescriptions for chronic illnesses” prescription form. This patient group is at a particularly high risk for complications and mortality caused by COVID‐19 because of its proclivity toward elderly people and those with comorbidities. We established a “drive‐through pharmacy” (Figure 2) for patients to refill their prescriptions outside the hospital to minimize interflows with potentially infected patients.
3.2.3. Partition of hospital zones during periods with many COVID‐19 cases
If the number of suspected or confirmed cases raises, functional or spatial units in a hospital, such as the ER, outpatient clinics, and inpatient wards, may be rearranged or modified to meet new and emergent medical needs. The following is an example of how to partition hospital zones.
KMUH comprises two main buildings: the old and new buildings. The old building complex contains three major wings (A, B, and C). The isolation ward and the “step‐down ward” (described in the “Step‐down ward” section as follows) are both located in the C wing on the 11th and 6th floors, respectively. The passageways between the B and C wings on these two floors are blocked to cut off all transportation. The two wards are connected with one designated elevator that only stops at these two floors. Apart from this, all staff members in these two wards are arranged to have as little interaction as possible with other personnel to minimize interflows with other clean zones.
Emergency department
We moved the triage area outside the ER for a preliminary screening of patients' history of TOCC (Figure 2). For ambulatory and noncritical patients at a risk of COVID‐19 infection, they are first arranged in the “outdoor screening area” that consists of tents (Figure 5). ER doctors then attend to the patient after wearing standard PPE for COVID‐19. All examinations and sample collections, including a throat swab, blood sample drawing, and portable radiography, are performed in these tents if the patient meets criteria for the COVID‐19 notification.
FIGURE 5.

Outdoor screening area built with tents to screen ambulatory noncritical patients at risk of COVID‐19 infection
Isolation ward
In February, a medical team consisting of infectious disease specialists, pulmonologists, and well‐trained residents from all departments began 10‐ or 14‐day rotations in the isolation ward to form a fixed‐team care model. During the duty period, these doctors do not directly interact with or share joint spaces with their colleagues from the same division. A hospital ward was evacuated and refitted into duty rooms for medical staff. If any confirmed cases appear during the duty period, all members engage in a 14‐day quarantine and self‐health management at home after their rotation.
Step‐down ward
Patients testing negative for COVID‐19 who require hospitalization are transferred to a “step‐down ward” for 48 hours before being transferred to general wards in various departments. This ward is meant to be a buffer zone for extended observation of patients' symptoms in case of a delayed presentation of COVID‐19.
Preparing for massive cases by expanding the red zone
To prepare for a massive volume of incoming patients from a community or hospital outbreak, we are prepared to transform the whole old building complex into an isolation zone if the epidemic spreads beyond containment. By that time, the B and C wings of the whole building will be assigned as red zones and room facilities will be rearranged for single patient isolation use. The A wing will become the green zone where medical staff work and rest after decontamination and removing their PPE in the red zone.
Restaurants
We close the dining area of hospital restaurants and allow only takeaways.
3.3. Third stage: Prevention of a systemic shutdown
On 29 February, the first hospital cluster of COVID‐19 in Taiwan was announced by the Central Epidemic Command Center (CECC). This nosocomial outbreak involved nine cases, and more than 60 health‐care workers were quarantined because of this incident. An emerging infectious disease outbreak in a hospital setting provokes enormous psychological stress among staff and causes man power shortage, which can be devastating during a pandemic. In response to this possible scenario, we adopted the following measures:
1. For all hospitalized patients, strict visitor restrictions are applied. We distribute one visitor card to each inpatient's family member or caretaker, and only one companion can enter at a time. People without visitor cards or staff ID cards are not allowed to enter the hospital building.
2. “Divided cabin, divided flow”: This is a term used by Taiwan's CECC and metaphorically compares a hospital to a ship. When a ship leaks, it is less likely to sink if it is divided into small cabins, which prevents immediate flooding. A similar scenario can occur during a hospital outbreak. “Divided flow,” indicates the aforementioned divided patient flows. As for “divided cabin,” we divide nursing staff in every nurse station into four or five working units. Every unit attends designated rooms and is assigned patients without alternation. In addition, the groups dine at different time schedules to prevent prolonged interaction periods without wearing facial masks. In case of a nosocomial outbreak, this method of “divided cabin” working units may prevent a mass quarantine and sudden massive loss of workforce.
3.4. Other interventions
3.4.1. Rapid influenza diagnostic test restriction
To minimize doctors' and paramedical staff members' risk of exposure to respiratory droplets, we suspended rapid influenza diagnostic tests (RIDTs) in all clinical settings. RIDTs are only available in the ER and in wards where sample collection can be performed in an adequately ventilated single room or negative pressure isolation room. We encourage all physicians to treat patients with influenza‐like illness (ILI) by using oseltamivir directly instead of performing RIDTs for diagnosis. Taiwan's CDC has expanded the duration of prescribing government‐funded free anti‐influenza antiviral drugs in response to the COVID‐19 outbreak and granted the use of oseltamivir for all patients with ILI in the end of April 2020.
3.4.2. Nebulizer therapy and intubation
Nebulizers may generate aerosol sources that can evaporate into tiny infectious droplet nuclei and be propelled over long distances. 11 Other aerosol generating procedures, such as noninvasive ventilation, high‐flow nasal cannula, bag‐valve‐mask (BVM) ventilation before intubation, and tracheal intubation contain particularly high risks of transmitting potentially viable respiratory viruses. 12 We restrict the use of all nebulizers and allow only metered dose inhalers. An early intubation strategy is adopted in patients with suspected or confirmed COVID‐19 to avoid BVM ventilation. A filter is connected to the equipment if BVM ventilation is necessary. All tracheal intubation is performed by an anesthesiologist equipped with a PAPR.
3.4.3. Active surveillance of suspected COVID‐19 cases inside the hospital
Because some COVID‐19 cases progress to severe pneumonia, we actively screen for cases whose pneumonia does not improve as expected after antibiotic treatment. With assistance from the IT department, we developed an automatic alert system to conduct daily syndromic and laboratory data screening of inpatients. The attending physician who receives alerts for potential cases must quickly reply to them. If COVID‐19 is suspected by the physician, a diagnostic test is performed. The patient is then transferred to an isolation ward before the final test results are available.
3.4.4. Perinatal setting
An algorithm and consensus document are established for the special concerns of perinatal settings, and a multidisciplinary team was created comprising a gynecologist, pediatrician, infectious disease specialist, and anesthesiologist. Vaginal deliveries are conducted in the isolation ward, and cesarean deliveries are performed in a designated operating room.
4. CONCLUSIONS
Because many hospitals and health‐care workers in Taiwan experienced the SARS epidemic in 2003, the current global COVID‐19 pandemic is a reminder of the devastating consequences of nosocomial outbreaks during epidemics of infectious diseases. The significance of maintaining a functional health‐care system during a pandemic cannot be overemphasized. Hospitals are the front line for delivering lifesaving treatment when society faces a serious and unpredictable emerging infectious disease. From the beginning, all efforts should be made to protect hospitals and medical staff from nosocomial infections because the collapse of the frontline health‐care system may result in a massive loss of life. A rapid response to an infectious disease outbreak is critical and requires the immediate execution of infection control measures nationally and in hospital settings. In KMUH, we took immediate action through consecutive interventions in response to the evolving COVID‐19 epidemic (Table 2). The measures taken by a medical center in Taiwan during the COVID‐19 pandemic may not be universally applicable for all medical facilities or health‐care systems in other countries. Nonetheless, the presented infection control methods have been practically executed and can be referenced or modified to fit each hospital's unique condition. An immediate response and implementation of infection control measures is critical to save lives during an epidemic both inside and outside the hospital.
TABLE 2.
Strategies and actions implemented by KMUH
| Date | Strategy/action |
|---|---|
| 22 January |
|
| 23 January |
|
| 24 January |
|
| 26 January |
|
| 27 January |
|
| 28 January |
|
| 29 January |
|
| 30 January |
|
| 3 February |
|
| 4 February |
|
| 7 February |
|
| 9 February |
|
| 18 February |
|
| 26 February |
|
| 12 March |
|
Abbreviations: CDC: Centers for Disease Control; ED: emergency department; ICU: intensive care unit; KMUH: Kaohsiung Medical University Hospital; NHI: National Health Insurance; PAPR: powered air‐purifying respirator; TOCC: travel, occupation, contacts, clusters.
Chang Y‐T, Lin C‐Y, Tsai M‐J, et al. Infection control measures of a Taiwanese hospital to confront the COVID‐19 pandemic. Kaohsiung J Med Sci. 2020;36:296–304. 10.1002/kjm2.12228
REFERENCES
- 1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu P, Zhu J, Zhang Z, Han Y, Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person‐to‐person transmission during the incubation period. J Infect Dis. 2020:jiaa077. 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int J Epidemiol. 2020:dyaa033. 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323:1488. 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellewell J, Abbott S, Gimma A, Bosse NI, Jarvis CI, Russell TW, et al. Feasibility of controlling COVID‐19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8(4):e488–e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Person B, Sy F, Holton K, Govert B, Liang A. Fear and stigma: The epidemic within the SARS outbreak. Emerg Infect Dis. 2004;10(2):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizumoto K, Chowell G. Estimating risk for death from 2019 novel coronavirus disease, China, January‐February 2020. Emerg Infect Dis. 2020;26(6). 10.3201/eid2606.200233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: A study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz J, King CC, Yen MY. Protecting health care workers during the COVID‐19 coronavirus outbreak‐lessons from Taiwan's SARS response. Clin Infect Dis. 2020:ciaa255. 10.1093/cid/ciaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7(4):e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]


