Abstract
Clinical and laboratory data on patients with coronavirus disease 2019 (COVID‐19) in Beijing, China, remain extremely limited. In this study, we summarized the clinical characteristics of patients with COVID‐19 from a designated hospital in Beijing. In total, 55 patients with laboratory‐confirmed SARS‐CoV‐2 infection in Beijing 302 Hospital were enrolled in this study. Demographic data, symptoms, comorbidities, laboratory values, treatments, and clinical outcomes were all collected and retrospectively analyzed. A total of 15 (27.3%) patients had severe symptoms, the mean age was 44.0 years (interquartile range [IQR], 34.0‐56.0), and the median incubation period was 7.5 days (IQR, 5.0‐11.8). A total of 26 (47.3%) patients had exposure history in Wuhan of less than 2 weeks, whereas 20 (36.4%) patients were associated with familial clusters. Also, eighteen (32.7%) patients had underlying comorbidities including hypertension. The most common symptom of illness was fever (45; 81.8%); 51 (92.7%) patients had abnormal findings on chest computed tomography. Laboratory findings showed that neutrophil count, percentage of lymphocyte, percentage of eosinophil, eosinophil count, erythrocyte sedimentation rate, albumin, and serum ferritin are potential risk factors for patients with a poor prognosis. A total of 26 patients (47.3%) were still hospitalized, whereas 29 (52.7%) patients had been discharged. Compared with patients in Wuhan, China, the symptoms of patients in Beijing are relatively mild. Older age, more comorbidities, and more abnormal prominent laboratory markers were associated with a severe condition. On the basis of antiviral drugs, it is observed that antibiotics treatment, appropriate dosage of corticosteroid, and gamma globulin therapy significantly improve patients' outcomes. Early identification and timely medical treatment are important to reduce the severity of patients with COVID‐19.
Keywords: clinical features, coronavirus disease 2019, designated Hospital in Beijing, SARS‐CoV‐2
1. INTRODUCTION
The deadly coronavirus disease 2019 (COVID‐19), which is caused by the severe acute respiratory coronavirus 2 (SARS‐CoV‐2), first emerged in Wuhan, Hubei province, China, in December 2019. 1 , 2 , 3 On 13 March, the World Health Organization declared that this novel coronavirus has become a global pandemic. The situation is ongoing and evolving rapidly; by 24 March 2020, COVID‐19 had spread to at least 189 countries and areas, with over 334 981 infected cases and 14 651 deaths worldwide. The recently published literature on the epidemic features of COVID‐19 mainly focuses on Wuhan, whereas clinical and laboratory data on COVID‐19 pneumonia in the capital of China, Beijing, are still extremely scarce. 4 Different from the situation of lack of medical care in the epidemic center—Wuhan at the early stage, these patients in Beijing have received relatively better medical care and treatment measures. Analyzing the clinical characteristics of patients in Beijing will be helpful to better understand this disease. In this study, we summarized the epidemiological and clinical features, laboratory data, treatment, and outcomes of patients admitted to the Fifth Medical Center of Chinese PLA General Hospital/Beijing 302 Hospital from 20 January 2020 to 15 February 2020, analyzing the related factors with the severity and outcomes of COVID‐19.
2. METHODS
2.1. Study design
We conducted a retrospective observational study on all patients with confirmed COVID‐19, who were admitted for care at the Beijing 302 Hospital from 20 January 2020 to 15 February 2020. In total, 55 patients were enrolled in this study. Laboratory confirmation of SARS‐CoV‐2 was made on the basis of the results of real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). Treatment was conducted in line with the diagnostic and treatment guidelines for SARS‐CoV‐2, issued by the Chinese National Health Committee (version 3‐5). 5
2.2. Ethical consideration
This study was approved by the ethics committee of the Beijing 302 Hospital/The Fifth Medical Center of PLA General Hospital.
2.3. Data sources
The clinical information of confirmed patients with COVID‐19 was recorded in detail in Beijing 302 Hospital, which is one of the three designated municipal hospitals in Beijing. Only patients with laboratory‐confirmed SARS‐CoV‐2 infection were enrolled in this study. 6 The data analyzed in this study include sex, age, disease severity, chest radiography findings, epidemiological history, the dates of illness onset, visits to clinical facilities, hospital admissions, the highest body temperature, underlying diseases, symptom information, laboratory values, treatment measures, and outcomes. The incubation period was defined as the period from exposure to the illness onset, based on the patients' description. Besides, the possibility of familial clusters, the index patients infecting other family members, was also investigated. The requirement for informed consent was waived due to a pressing need to gather data on this emerging pathogen in Beijing. The information of each patient was accurately and carefully checked by at least two researchers independently.
2.4. Clinical diagnostic classification
On the basis of diagnostic and treatment guideline for SARS‐CoV‐2, issued by Chinese National Health Committee (version 3‐5), 5 patients with COVID‐19 were classified into four types: mild type with slight clinical symptoms, but no imaging presentations of pneumonia; common type with fever, respiratory symptoms, and imaging presentations of pneumonia; severe type with any of the following: respiratory distress with respiratory frequency ≥30 times/min, pulse oximeter oxygen saturation ≤ 93% at rest, or oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction) ≤300 mm Hg (1 mm Hg = 0.133kPa); and critically severe type with any of the following: respiratory failure needing mechanical ventilation, shock, or a combination with other organ failure needing intensive care unit. 7 In this study, we classified patients with mild and common symptoms into the nonsevere group and severe and critically severe type into the severe group.
2.5. Laboratory confirmation and treatment
Sputum and throat swab specimens collected from all patients at admission were tested by real‐time RT‐PCR for SARS‐CoV‐2 RNA within 3 hours. 8 Virus detection was repeated twice every 24 hours. Laboratory tests were conducted at admission, including a complete blood count, coagulation profile, serum biochemistry (including renal and liver function, creatine kinase, lactate dehydrogenase, and electrolytes), myocardial enzymes, interleukin‐6 (IL‐6), serum ferritin, urine routine, blood gas, and identification of other respiratory pathogens, such as influenza A virus (H1N1, H3N2, and H7N9), influenza B virus, respiratory syncytial virus (RSV), parainfluenza virus, and adenovirus. Most patients received antiviral treatment with interferon‐alpha inhalation (50 μg twice daily), lopinavir and ritonavir (400 mg twice daily and 100 mg twice daily, respectively), and arbidol (200 mg twice daily). Corticosteroid (40‐80 mg/d) and gamma globulin (15‐20 g/d) was given as a combined regimen for 3 to 5 days if patient's resting respiratory rate was more than 30 per minute, or oxygen saturation was below 93% without oxygen, or multiple pulmonary lobes showed more than 50% progression of disease in 48 hours on imaging. Patients infected with SARS‐CoV‐2 were discharged from the hospital on the basis of abatement of fever, improved evidence on chest radiography, as well as the negative results for SARS‐CoV‐2 nucleic acid of two real‐time RT‐PCR tests taken within a gap of 24 hours.
2.6. Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR). Categorical variables were summarized as the counts and percentages in each category. Wilcoxon rank‐sum tests were applied to continuous variables, and χ 2 tests and Fisher's exact tests were used for categorical variables as appropriate. All analyses were done with SPSS software, version 22.0.
3. RESULTS
3.1. Epidemiological characteristics
By 15 February 2020, a total of 55 patients infected with SARS‐CoV‐2 in Beijing 302 Hospital were enrolled in this study, with 40 (72.7%) patients being categorized as nonsevere patients and 15 (27.3%) as severe cases on admission (Table 1). The median age for all patients was 44 years (IQR, 34‐56 years). Also, 4 patients (7.3%) aged between 0 and 18 years, 20 (36.4%) aged between 19 and 40 years, 21 (38.2%) aged between 41 and 65 years, and 10 (18.2%) aged 66 years and older. Of these patients, 31 (56.4%) were male, whereas 24 (43.6%) were female. A total of 26 (47.3%) patients had exposure history in Wuhan of less than 2 weeks and 6 (10.9%) patients lived in Wuhan and came from Wuhan to Beijing. A total of 19 (34.5%) patients could provide the clues of close contact with the confirmed patients with COVID‐19, 12 (63.2%) of them were infected by their family members, and 7 (36.8%) were infected by other patients or their colleagues in workplaces. However, four (7.3%) patients could not provide the clues of close contact with the patients with COVID‐19, and no patient had been exposed to the Huanan seafood market. Of 55 patients, 20 (36.4%) were associated with familial clusters (Table 1).
Table 1.
Characteristics of 55 patients with COVID‐19 in Beijing 302 Hospital
| Disease severity | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All patients (n = 55) | Nonsevere (n = 40) | Severe(n = 15) | P valuea | Hospitalization (n = 26) | Discharged (n = 29) | P valueb |
| Median (interquartile) age, y | 44.0 (34.0, 56.0) | 39.5 (32.0, 51.8) | 67.0 (46.0, 77.0) | .000 | 54.5 (39.0, 74.0) | 37.0 (28.0, 46.5) | .001 |
| Age groups, y | |||||||
| 0‐18 | 4 (7.3) | 4 (10.0) | 0 (0.0) | 1 (3.8) | 3 (10.3) | ||
| 19‐40 | 20 (36.4) | 17 (42.5) | 3 (20.0) | 7 (26.9) | 13 (44.8) | ||
| 41‐65 | 21 (38.2) | 17 (42.5) | 4 (26.7) | 9 (34.6) | 12 (41.4) | ||
| ≥66 | 10 (18.2) | 2 (5.0) | 8 (53.3) | 9 (34.6) | 1 (3.4) | ||
| Sex | .781 | .368 | |||||
| Male | 31 (56.4) | 23 (57.5) | 8 (53.3) | 13 (50.0) | 18 (62.1) | ||
| Female | 24 (43.6) | 17 (42.5) | 7 (46.7) | 13 (50.0) | 11 (37.9) | ||
| Epidemiological history | .032 | .005 | |||||
| Exposure history in Wuhan <2 wk | 26 (47.3) | 23 (57.5) | 3 (20.0) | 6 (23.1) | 20 (69.0) | ||
| Come from Wuhan | 6 (10.9) | 4 (10.0) | 2 (13.3) | 3 (11.5) | 3 (10.3) | ||
| Close contacts | 19 (34.5) | 12 (30.0) | 7 (46.7) | 14 (53.8) | 5 (17.2) | ||
| Others | 4 (7.3) | 1 (2.5) | 3 (20.0) | 3 (11.5) | 1 (3.4) | ||
| Familial cluster | 20 (36.4) | 17 (42.5) | 3 (20.0) | .122 | 10 (38.5) | 10 (34.5) | .759 |
| Comorbidities | |||||||
| Any | 18 (32.7) | 10 (25.0) | 8 (53.3) | .046 | 12 (46.2) | 6 (20.7) | .044 |
| Hypertension | 8 (14.5) | 2 (5.0) | 6 (40.0) | .001 | 7 (26.9) | 1 (3.4) | .014 |
| Diabetes | 5 (9.1) | 3 (7.5) | 2 (13.3) | .503 | 3 (11.5) | 2 (6.9) | .550 |
| Respiratory diseases | 4 (7.3) | 3 (7.5) | 1 (6.7) | .916 | 1 (3.8) | 3 (10.3) | .354 |
| Thyroid disease | 3 (5.5) | 3 (7.5) | 0 (0.0) | .275 | 2 (7.7) | 1 (3.4) | .489 |
| Chronic liver disease | 3 (5.5) | 1 (2.5) | 2 (13.3) | .115 | 2 (7.7) | 1 (3.4) | .489 |
| Chronic kidney disease | 1 (1.8) | 0 (0.0) | 1 (6.7) | .099 | 0 (0.0) | 1 (3.4) | .339 |
| Cardiovascular diseases | 1 (1.8) | 0 (0.0) | 1 (6.7) | .099 | 0 (0.0) | 1 (3.4) | .339 |
| Highest temperature before admission, °C | .701 | .401 | |||||
| <37.3 | 8 (14.5) | 7 (17.5) | 1 (6.7) | 2 (7.6) | 6 (20.7) | ||
| 37.3‐38.0 | 21 (38.2) | 14 (35.0) | 7 (46.7) | 12 (46.2) | 9 (31.0) | ||
| 38.01‐39.0 | 19 (34.5) | 14 (35.0) | 5 (33.3) | 8 (30.8) | 11 (37.9) | ||
| >39.0 | 5 (9.1) | 4 (10.0) | 1 (6.7) | 3 (11.5) | 2 (6.9) | ||
| Missing | 2 (3.6) | 1 (2.5) | 1 (6.7) | 2 (7.7) | 0 (0.0) | ||
| Symptom | |||||||
| Cough | 26 (47.3) | 17 (42.5) | 9 (60.0) | .247 | 15 (57.7) | 11 (37.9) | .143 |
| Fatigue | 14 (25.5) | 10 (25.0) | 4 (26.7) | .899 | 8 (30.8) | 6 (20.7) | .392 |
| Expectoration | 13 (23.6) | 8 (20.0) | 5 (33.3) | .300 | 8 (30.8) | 5 (17.2) | .238 |
| White sputum | 9 (16.4) | 5 (12.5) | 4 (26.7) | .206 | 7 (26.9) | 2 (6.9) | .045 |
| Yellow sputum | 3 (5.5) | 1 (2.5) | 2 (13.3) | .115 | 2 (7.7) | 1 (3.4) | .489 |
| Muscle ache | 10 (18.2) | 7 (17.5) | 3 (20.0) | .830 | 7 (26.9) | 3 (10.3) | .112 |
| Sore throat | 8 (14.5) | 6 (15.0) | 2 (13.3) | .876 | 3 (11.5) | 5 (17.2) | .549 |
| Anorexia | 7 (12.7) | 4 (10.0) | 3 (20.0) | .322 | 6 (23.1) | 1 (3.4) | .029 |
| Dizziness | 6 (10.9) | 4 (10.0) | 2 (13.3) | .724 | 4(15.4) | 2 (6.9) | .313 |
| Headache | 6 (10.9) | 4 (10.0) | 2 (13.3) | .724 | 3 (11.5) | 3 (10.3) | .887 |
| Shortness of breath | 5 (9.1) | 1 (2.5) | 4 (26.7) | .005 | 5 (19.2) | 0 (0.0) | .013 |
| Chills | 4 (7.3) | 2 (5.0) | 2 (13.3) | .289 | 3 (11.5) | 1 (3.4) | .249 |
| Dry cough | 3 (5.5) | 3 (7.5) | 0 (0.0) | .275 | 1 (3.8) | 2 (6.9) | .619 |
| Dyspnea | 3 (5.5) | 1 (2.5) | 2 (13.3) | .115 | 2 (7.7) | 1 (3.4) | .489 |
| Skin rash | 1 (1.8) | 1 (2.5) | 0 (0.0) | .537 | 0 (0.0) | 1 (3.4) | .339 |
| Nasal congestion | 1 (1.8) | 1 (2.5) | 0 (0.0) | .537 | 0 (0.0) | 1 (3.4) | .339 |
| Diarrhea | 1 (1.8) | 1 (2.5) | 0 (0.0) | .537 | 0 (0.0) | 1 (3.4) | .339 |
| Abnormalities on chest CT | .916 | .354 | |||||
| No | 4 (7.3) | 3 (7.5) | 1 (6.7) | 1 (3.8) | 3 (10.3) | ||
| Yes | 51 (92.7) | 37 (92.5) | 14 (93.3) | 25 (96.2) | 26 (89.7) | ||
| Abnormalities of the lung at admission | .076 | .179 | |||||
| No lesion | 3 (5.5) | 3 (7.5) | 0 (0.0) | 0 (0.0) | 3 (10.3) | ||
| Unilateral lesion | 8 (14.5) | 8 (20.0.0) | 0 (0.0) | 3 (11.5) | 5 (17.2) | ||
| Bilateral lesion | 44 (80.0) | 29 (72.5) | 15 (100.0) | 23 (88.5) | 21 (72.4) | ||
| Lung inflammation at admission | .026 | .005 | |||||
| No inflammation | 16 (29.1) | 14 (35.0) | 2 (13.3) | 3 (11.5) | 13 (44.8) | ||
| A little inflammation | 7 (12.7) | 7 (17.5) | 0 (0.0) | 2 (7.7) | 5 (17.2) | ||
| Massive inflammation | 32 (58.2) | 19 (47.5) | 13 (86.7) | 21 (80.8) | 11 (37.9) | ||
| Type of lesion at admission | .201 | .011 | |||||
| No clear inflammatory changes | 16 (29.1) | 14 (35.0) | 2 (13.3) | 3 (11.5) | 13 (44.8) | ||
| Atypical inflammatory changes | 30 (54.5) | 21 (52.5) | 9 (60.0) | 16 (61.5) | 14 (48.3) | ||
| Typical ground‐glass opacity | 9 (16.4) | 5 (12.5) | 4 (26.7) | 7 (26.9) | 2 (6.9) | ||
| Incubation period, d | 7.5 (5.0, 11.8) | 7.0 (4.5, 12.5) | 8.0 (6.0, 10.0) | .908 | 7.5 (6.0, 10.0) | 7.5 (4.0, 15.8) | .907 |
| Duration from illness onset to the first admission, d | 4.0 (3.0, 7.0) | 2.0 (1.0, 4.0) | 3.5 (2.0, 6.3) | .065 | 4.0 (1.5, 6.0) | 1.0 (0.0, 3.0) | .004 |
| Duration from other hospitals to designated hospitals, d | 2.0 (1.0, 5.0) | 4.0 (1.0, 6.3) | 6.5 (4.0, 9.5) | .033 | 6.0 (4.0, 8.0) | 3.0 (2.0, 6.0) | .009 |
Note: Values are the number (percentages) of patients unless stated otherwise.
Abbreviations: COVID‐19, coronavirus disease 2019; CT, computed tomography.
P value for the comparison between nonsevere patients and severe patients.
P value for the comparison between hospitalized patients and discharged patients.
Of the 40 patients with nonsevere symptoms, 23 (57.5%) were male and 17 (42.5%) were female. Also, 4 (10.0%) patients aged less than 18 years, 17 (42.5%) aged between 19 and 40 years, 17 (42.5%) aged between 41 and 65 years, and 2 (5.0%) aged more than 65 years. Of the 15 severe patients, 8 (53.3%) were male, whereas 7 (46.7%) were female. Furthermore, three (20.0%) patients aged between 19 and 40 years, four (26.7%) aged between 41 and 65 years, and eight (53.3%) aged more than 66 years. The median age of severe patients is significantly older than nonsevere patients (67.0 vs 39.5; P < .001), and no obvious differences of disease severity were observed between male and female patients (P = 0.781) (Table 1).
Among the patients who could provide the exact date of close contact with someone confirmed or suspected with SARS‐CoV‐2 infection, the median incubation period from exposure to symptoms was 7.5 days (IQR, 5.0‐11.8 days). The median time from the onset of symptoms to first hospital admission was 4.0 days (IQR, 3.0‐7.0 days) (Table 1).
3.2. Clinical features
Of 55 patients, 18 (32.7%) had at least one underlying comorbidity; chronic diseases, such as hypertension (8; 14.5%), diabetes (5; 9.1%), respiratory disease (4; 7.3%), thyroid disease (3; 5.5%), chronic liver disease (3; 5.5%), chronic kidney disease (1; 1.8%), and cardiovascular disease (1; 1.8%), were the most common identified comorbidities (Table 1). Among the 15 severe patients, 8 (53.3%) had at least one underlying comorbidity: 6 (40.0%) patients had hypertension, 2 (13.3%) had diabetes, 2 (13.3%) had chronic liver disease, and 1 each had respiratory disease (6.7%), chronic kidney disease (6.7%), and cardiovascular disease (6.7%).
Of the 53 patients with the body temperature records, 45 (81.8%) patients' highest body temperature before hospital admission was higher than 37.3°C, suggesting that fever is still one of the typical symptoms of COVID‐19. Besides fever, the common symptoms of the enrolled patients at admission were cough (26; 47.3%), fatigue (14; 25.5%), expectoration (13; 23.6%), muscle ache (10; 18.2%), sore throat (8; 14.5%), anorexia (7; 12.7%), dizziness (6; 10.9%), headache (6; 10.9%), shortness of breath (5; 9.1%), chills (4; 7.3%), dry cough (3; 5.5%), and dyspnea (3; 5.5%). According to imaging examination, the majority of the patients (51; 92.7%) had abnormal lung findings, with 44 (80%) patients showing bilateral pneumonia and 8 (14.5%) showing unilateral lesions. A total of 30 (54.5%) patients exhibited atypical inflammatory changes, 16 (29.1%) showed no clear inflammatory changes, and 9 (16.4%) showed typical ground‐glass opacity (Figure 1). A total of 32 (58.2%) patients showed a substantial lung inflammation, 16 (29.1%) showed no inflammation, and 7 (12.7%) showed a little inflammation in the lungs.
Figure 1.
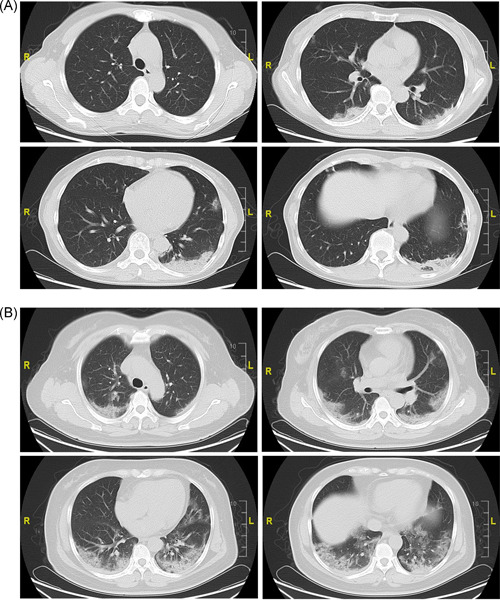
Chest computed tomographic images of patients with COVID‐19. A, Computed tomography images of a 55‐year‐old woman with nonsevere symptoms, showing patchy consolidation in both lungs. B, Computed tomography images of a 50‐year‐old woman with severe symptoms, showing bilateral ground‐glass opacity and consolidation on day 4 after fever and cough onset. COVID‐19, coronavirus disease 2019
Further analysis found that compared with nonsevere patients, severe patients were more likely to suffer from shortness of breath (26.7% vs 2.5%) and lung inflammation, especially massive lung inflammation (86.7% vs 47.5%) (P < .05) at admission. Also, hypertension (40.0% vs 5.0%), untimely treatment (duration from illness onset to the first admission and the duration from other hospitals to designated hospitals) could exacerbate the COVID‐19 disease severity (P < .05). In addition, age (37.0 vs 54.5), hypertension (3.4% vs 26.9%), anorexia (3.4% vs 23.1%), shortness of breath (0.0% vs 19.2%), lung inflammation at admission (especially massive inflammation [37.9% vs 80.8%]), type of lesion at admission (especially typical ground‐glass opacity [6.9% vs 26.9%]), duration from illness onset to the first admission (days) (1.0 vs 4.0), and duration from other hospitals to designated hospitals (days) (3.0 vs 6.0) could significantly influence the clinical outcomes (discharge or nondischarge) of patients (P < .05).
3.3. Laboratory parameters
To determine the major clinical features that appeared during COVID‐19 progression, the dynamic changes in tested clinical laboratory parameters, including blood routine, blood chemistry, liver function, kidney function, urine routine, and blood gas, were tracked from 20 January 2020 to 15 February 2020. We collected the results of laboratory examinations of all patients since admission. During hospitalization, the same laboratory index may be required for multiple inspections at different time points, based on patients' conditions. As long as there is an abnormality in the specific laboratory index (increase or decrease), we consider that this index for the patient is abnormal. During the hospitalization of 55 patients from 20 January 2020 to 15 February 2020, a total of 152 laboratory parameters were found to be abnormal for all patients; 123 abnormalities occurred in nonsevere patients, 139 abnormalities in patients with severe symptoms, 146 in hospitalized patients, and 127 in discharged patients (Table 2).
Table 2.
Top 60% of significant abnormal laboratory parameters of 55 patients with COVID‐19
| Disease severity | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All patients (n = 55) | Nonsevere (n = 40) | Severe (n = 15) | P valuea | Hospitalization (n = 26) | Discharged (n = 29) | P valueb |
| Blood routine | |||||||
| Percentage of neutrophil | 44 (80.0) | 29 (72.5) | 15 (100.0) | .025 | 25 (96.1) | 19 (65.5) | .006 |
| Percentage of lymphocyte | 40 (72.7) | 25 (62.5) | 15 (100.0) | .005 | 24 (92.3) | 16 (55.1) | .002 |
| Percentage of eosinophil | 40 (72.7) | 25 (62.5) | 15 (100.0) | .005 | 24 (92.3) | 16 (55.1) | .000 |
| Neutrophil count | 37 (67.2) | 22 (55.0) | 15 (100.0) | .010 | 24 (92.3) | 13 (44.8) | .000 |
| Eosinophil count | 36 (65.4) | 21 (52.5) | 15 (100.0) | .001 | 23 (88.4) | 13 (44.8) | .001 |
| Leucocyte | 35 (63.6) | 22 (55.0) | 13 (86.7) | .030 | 21 (80.7) | 14 (48.2) | .024 |
| Infection‐related biomarkers | |||||||
| Erythrocyte sedimentation rate | 40 (72.7) | 25 (62.5) | 15 (100.0) | .005 | 24 (92.3) | 16 (55.1) | .019 |
| Liver function | |||||||
| Prealbumin | 34 (61.8) | 21 (52.5) | 13 (86.6) | .029 | 23 (88.4) | 11 (37.9) | .000 |
| Albumin | 33 (60.0) | 18 (45.0) | 15 (100.0) | .000 | 23 (88.4) | 10 (34.4) | .001 |
| Iron metabolism | |||||||
| Serum ferritin | 35 (63.6) | 20 (50.0) | 15 (100.0) | .000 | 24 (92.3) | 11 (37.9) | .000 |
Note: Values are numbers (percentages).
Abbreviation: COVID‐19, coronavirus disease 2019.
P value for the comparison between nonsevere patients and severe patients.
P value for the comparison between hospitalized patients and discharged patients.
The number of top 60% abnormal parameters among all patients was 17 (Figure 2A), in nonsevere patients, it was 7 (Figure 2B), in severe patients, it was 45 (Figure 2C), in hospitalized patients, it was 34 (Figure 3A), and in discharged patients, it was 3 (Figure 3B). The top five abnormal laboratory parameters for all patients were the percentage of neutrophil (80.0%, representing frequency), IL‐6 (74.5%), iron (74.5%), the percentage of lymphocyte (72.7%), lactic acid (72.7%), the percentage of eosinophil (72.7%), and the erythrocyte sedimentation rate (ESR) (72.7%) (Figure 2A). The top five abnormal test indicators for nonsevere patients were IL‐6 (72.5%), iron (72.5%), the percentage of neutrophil (72.5%), lactic acid (67.5%), and the percentage of lymphocyte (62.5%) (Figure 2B). The most abnormal parameters for severe patients were albumin (100.0%), the percentage of lymphocyte (100.0%), pro‐brain natriuretic peptide (100.0%), the percentage of eosinophil (100.0%), eosinophil count (100.0%), ESR (100.0%), serum ferritin (100.0%), the percentage of neutrophil (100.0%), and neutrophil count (100.0%) (Figure 2C). The most abnormal parameters of hospitalized patients were the percentage of neutrophil (96.2%), the percentage of lymphocyte (92.3%), lactic acid (92.3%), the percentage of eosinophil (92.3%), ESR (92.3%), serum ferritin (92.3%), and neutrophil count (92.3%) (Figure 3A). The top five abnormal indicators of discharged patients were IL‐6 (69.0%), iron (69.0%), the percentage of neutrophil (65.5%), the percentage of lymphocyte (55.2%), and lactic acid levels (55.2%) (Figure 3B). Further analysis found that the abnormality ratio of 47 parameters of severe patients was higher than that of nonsevere patients (P < .05), and the abnormality ratio of 33 indicators of hospitalized patients was higher than that of discharged patients (P < .05). The potential risk factors of the neutrophil count, the percentage of lymphocyte, the percentage of eosinophil, eosinophil count, ESR, albumin, and serum ferritin could help clinicians to identify patients with a poor prognosis at an early stage (Table 2 and Table S1).
Figure 2.
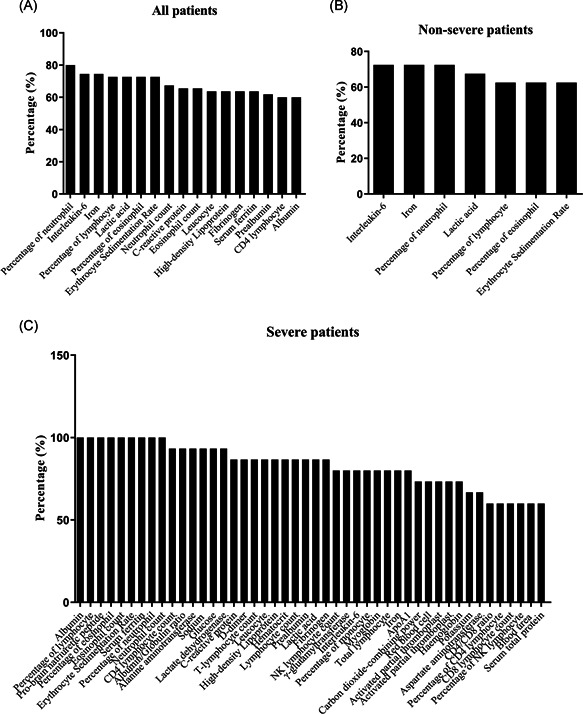
Laboratory findings in patients with COVID‐19. A, Top 60% of the abnormal laboratory parameters among all patients with COVID‐19. B, Top 60% of the abnormal laboratory parameters in nonsevere patients with COVID‐19. C, Top 60% of abnormal parameters in severe patients with COVID‐19. COVID‐19, coronavirus disease 2019
Figure 3.
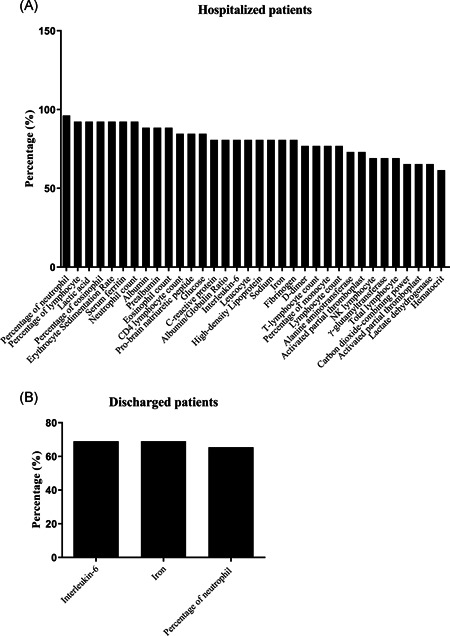
Laboratory findings in hospitalized and discharged patients with COVID‐19. A, Top 60% of abnormal laboratory parameters among hospitalized patients. B, Top 60% of abnormal parameters among discharged patients. COVID‐19, coronavirus disease 2019
3.4. Treatment and clinical outcomes
The clinical treatment and outcomes of the 55 patients are shown in Table 3. As of 15 February 2020, 26 patients (47.3%) were still hospitalized, whereas a total of 29 patients (52.7%) had been discharged. As compared with nonsevere cases, significantly more severe cases received mechanical ventilation (noninvasive: 33.3% vs 0%, P = .001; invasive: 20.0% vs 0%, P = .017). Only one patient (12.5%) of those who received mechanical ventilation had been discharged, whereas the other seven patients (87.5%) were still hospitalized. A total of 48 (87.3%) patients received antiviral therapy and 51 (92.7%) patients were given interferon‐alpha inhalation. A total of 43 (78.2%) cases received lopinavir/ritonavir treatment and 36 (65.5%) patients were given lopinavir/ritonavir plus interferon‐alpha inhalation treatment. A total of 29 (52.7%) patients were given empirical antibiotic treatment, 25 (45.5%) were given systematic corticosteroid treatment, and 22 (40.0%) received gamma globulin treatment. Moreover, blood purification therapy was adopted in three (20.0%) severe cases, but nonsevere cases did not receive this treatment (P = .004). Chinese medicine was given to six (10.9%) cases and one (1.8%) patient received convalescent plasma therapy. On the basis of antiviral drugs, it is observed that antibiotics treatment, appropriate dosage of corticosteroid, and gamma globulin therapy significantly improve patients' outcomes (P < .05).
Table 3.
Treatments and outcomes of 55 patients with COVID‐19
| Treatments | All patients (n = 55) | Disease severity | Outcomes | ||||
|---|---|---|---|---|---|---|---|
| Nonsevere (n = 40) | Severe (n = 15) | P valuea | Hospitalization (n = 26) | Discharged (n = 29) | P valueb | ||
| Interferon alpha inhalation | 51 (92.7) | 39 (97.5) | 12 (80.0) | .026 | 24 (92.3) | 27 (93.1) | .910 |
| Antiviral therapy | 48 (87.3) | 35 (87.5) | 13 (86.7) | .934 | 24 (92.3) | 24 (82.8) | .426 |
| Lopinavir/ritonavir | 43 (78.2) | 32 (80.0) | 11 (73.3) | .594 | 18 (69.2) | 25 (86.2) | .192 |
| Lopinavir/ritonavir + interferon alpha inhalation | 36 (65.5) | 26 (65.0) | 10 (66.7) | .908 | 18 (69.2) | 18 (62.1) | .577 |
| Antibiotics | 29 (52.7) | 17 (42.5) | 12 (80.0) | .013 | 19 (73.1) | 10 (34.5) | .004 |
| Corticosteroid | 25 (45.5) | 14 (35.0) | 11 (73.3) | .011 | 19 (73.1) | 6 (20.7) | .000 |
| Gamma globulin | 22 (40.0) | 12 (30.0) | 10 (66.7) | .013 | 17 (65.4) | 5 (17.2) | .000 |
| Mechanical ventilation | 8 (14.5) | 0 (0.0) | 8 (53.3) | 7 (26.9) | 1 (3.4) | .02 | |
| Noninvasive | 5 (9.1) | 0 (0.0) | 5 (33.3) | .001 | 5 (19.2) | 0 (0.0) | .013 |
| Invasive | 3 (5.5) | 0 (0.0) | 3 (20.0) | .017 | 2 (7.7) | 1 (3.4) | .598 |
| Chinese medicine | 6 (10.9) | 5 (12.5) | 1 (6.7) | .537 | 1 (3.8) | 5 (17.2) | .112 |
| Arbidol | 5 (9.1) | 3 (7.5) | 2 (13.3) | .503 | 5 (19.2) | 0 (0.0) | .013 |
| Arbidol + interferon‐alpha inhalation | 5 (9.1) | 3 (7.5) | 2 (13.3) | .503 | 5 (19.2) | 0 (0.0) | .013 |
| Blood purification | 3 (5.5) | 0 (0.0) | 3 (20.0) | .004 | 2 (7.7) | 1 (3.4) | .489 |
| Ribavirin | 1 (1.8) | 1 (2.5) | 0 (0.0) | .537 | 1 (3.8) | 0 (0.0) | .286 |
| Aciclovir | 1 (1.8) | 1 (2.5) | 0 (0.0) | .537 | 0 (0.0) | 1 (3.4) | .339 |
| Convalescent plasma | 1 (1.8) | 0 (0.0) | 1 (6.7) | .099 | 1 (3.8) | 0 (0.0) | .286 |
Note: Values are the number (percentages) of patients.
Abbreviation: COVID‐19, coronavirus disease 2019.
P value for the comparison between nonsevere patients and severe patients.
P value for the comparison between hospitalized patients and discharged patients.
4. DISCUSSION
As of 24 March 2020, SARS‐CoV‐2 infection had spread to 189 countries and areas, with more than 334 thousand patients and more than 14 000 deaths worldwide. The number of infected patients is still increasing rapidly. The clinical characteristics of early cases of COVID‐19 in Wuhan City have been immensely reported. 8 , 9 , 10 , 11 , 12 The infection and clinical characteristics of patients in Beijing, the capital of China, have attracted much attention, but a few of them have been reported. Among the 55 analyzed patients, there were many cases (20; 36.4%) of family clustering in this study. There is no doubt that SARS‐CoV‐2 has a strong human‐to‐human transmission characteristic, as previously reported. 13 , 14
More than half of the patients in Beijing 302 Hospital are male (31; 56.4%), with a wide range of age, including a 3‐year‐old boy and an 85‐year‐old lady. Compared with the initially reported high proportion of severe patients and high fatality in Wuhan, the symptoms of patients in Beijing are relatively mild, and there is only one death caused by claustrophobia, which is excluded from this study. 15
Here, we studied the effect of seven complications on the severity and outcomes of patients in detail. Whether suffering from hypertension is the only risk factor for severe patients and nonsevere patients, as well as for hospitalized patients and discharged patients are still need further investigation. Of the 55 patients with positive SARS‐CoV‐2 nucleic acid test, 45 (81.8%) had a body temperature of over 37.3°C and 51 (92.7%) had abnormal chest computed tomography (CT) findings, suggesting that abnormal body temperature and lung CT are still important indicators for COVID‐19 diagnosis.
Among the screened abnormal laboratory tests, indexes including blood routine, T cell subsets, infection‐related biomarkers, liver function, glucose, apolipoprotein A1, serum ferritin, myocardial enzyme, and kidney function were significantly different between the severe patients and nonsevere patients. Moreover, most of these laboratory parameters were statistically different between hospitalized and discharged patients (Table 2 and Table S1). Thus, these laboratory parameters could be used as the potential predictors of the severity and prognosis of SARS‐CoV‐2‐infected patients. Other studies have also shown that a significant reduction in the total number of lymphocytes, which indicates that coronavirus consumes many immune cells and inhibits the cellular immune function of the human body. The damage of T lymphocytes may be an important factor in the deterioration of patients with SARS‐CoV, 16 and the low absolute lymphocyte value can be used as an important reference index for clinical diagnosis of SARS‐CoV‐2 infection. 12 Significantly higher levels of D‐dimer, C‐reactive protein, and procalcitonin were associated with severe patients, compared with nonsevere patients. 17
In terms of treatment methods, mechanical ventilation was used in eight severe patients (five of them were noninvasive and three of them were invasive). A total of 48 (87.3%) patients received antiviral therapy, 51 (92.7%) patients were given interferon‐alpha inhalation, and 43 (78.2%) patients received lopinavir/ritonavir therapy. Also, 5 (9.1%) patients used arbidol, 1 (1.8%) used ribavirin or aciclovir, 5 (9.1%) used arbidol plus interferon‐alpha inhalation, and 36 (65.5%) used lopinavir/ritonavir plus interferon‐alpha inhalation. It is worth noting that more severe patients (12; 80.0%) received antibiotics, compared with nonsevere patients (17; 42.5%) (P < .05), which is possibly due to more coinfection of bacteria and fungi in severe patients. In addition, corticosteroid, gamma globulin, and blood purification were given priority in severe patients instead of nonsevere patients (P < .05).
The treatment of COVID‐19 is now in the exploratory stage all over the world, with several ongoing antiviral drug clinical trials. No therapeutics have yet been proven effective for the treatment of severe illness caused by SARS‐CoV‐2. Moreover, it is reported that lopinavir/ritonavir may have the potential to treat SARS infection. 18 According to the guidelines and suggestions of the Chinese National Health Committee, most of the SARS‐CoV‐2‐infected patients in this study were treated with lopinavir/ritonavir, which may be one of the main reasons for the better prognosis of patients in our hospital than those in Wuhan. However, the complications of current antiviral drugs have been reported by clinical studies. A trial of lopinavir/ritonavir in adults hospitalized with severe COVID‐19 was conducted by a previous study. They found that gastrointestinal adverse events, such as nausea, vomiting, diarrhea, anorexia, skin eruptions and hepatotoxicity were more common in lopinavir/ritonavir group than in the standard‐care group. 19 In the current study, diarrhea was found in the patients treated with lopinavir/ritonavir. On the basis of diagnostic and treatment guidelines for SARS‐CoV‐2, issued by the Chinese National Health Committee (version 3‐7), 5 the treatment period of lopinavir/ritonavir should not be more than 10 days. Ribavirin is a guanosine analog antiviral drug that has been used to treat several viral infections, including RSV, hepatitis C virus, SARS‐CoV, and Middle East respiratory syndrome‐CoV. 20 , 21 However, ribavirin could reduce hemoglobin concentrations, which is an undesirable side effect for patients with respiratory disorders. 22 Corticosteroids may prolong viral replication and increase the risk of secondary infection; thus, they should not be used routinely for treating patients with COVID‐19. They are recommended for COVID‐19 patients with other indications such as chronic obstructive pulmonary disease exacerbations and septic shock. 23 Several potential drugs, such as remdesivir, chloroquine phosphate, and cepharanthine, are believed to contribute to the treatment of COVID‐19. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Presently, there are no vaccine and effective anti‐SARS‐CoV‐2 drugs available to treat critically ill patients. Therefore, it is urgent to investigate the safety and efficacy of potential drugs for the treatment of COVID‐19 and develop new effective antiviral drugs against SARS‐CoV‐2.
There are several limitations to this study. First, only 55 patients with confirmed SARS‐CoV‐2 were included. A number of patients were continually being admitted to hospital as data were being collected; thus, we obtained data on most, but not all patients with laboratory‐confirmed infection in Beijing 302 Hospital during the study period. It would be better to include as many patients as possible in Beijing, and a larger cohort study would help us to further define the demographic data, symptoms, comorbidities, laboratory values, treatments, and clinical outcomes. Second, the kinetics of viral load and antibody titers were not demonstrated in this study. Third, we no doubt missed patients who were asymptomatic. Fourth, a number of patients had not been discharged at the time of study submission, so we were unable to estimate either the case mortality rate or the predictors of fatality. More effort should be made to answer these questions in future studies. However, the data in this study permit an early assessment of the epidemiological and clinical characteristics of COVID‐19 in Beijing.
Although the information on COVID‐19 has increased rapidly since the emergence of SARS‐CoV‐2, many problems still remain unresolved. The clinical manifestation of COVID‐19 can present as an asymptomatic carrier state, acute respiratory disease, and pneumonia. During our study period, there were no data reported on asymptomatic infections in Beijing 302 Hospital. Our understanding of this deadly coronavirus has gradually deepened. The study by Wang et al demonstrated that asymptomatic carriers occurred more often in middle‐aged people who had close contact with infected family members. The majority of the cases developed to be mild and ordinary COVID‐19 in hospital. 32 However, another study found that the 15 asymptomatic cases who were tested positive by RT‐PCR assay were either family members or close contacts with certain confirmed cases either symptomatic or asymptomatic, consisting of 5 males and 10 females. And the age ranged from 5 to 76 years. 1 Zou et al 33 reported that the viral load detected in asymptomatic patients was similar to that found in symptomatic patients; however, the viral loads from patients with severe diseases were higher than those in patients with mild‐to‐moderate presentations. As SARS‐CoV‐2 can be detected in the asymptomatic individual, the prophylactic or pre‐emptive use of effective antiviral agents to reduce the viral load and decrease the risk of virus spread from asymptomatic carriers may help to control the spread of COVID‐19. For controlling this disease, early detection and isolation of the confirmed cases are essential, followed by tracing and screening their contacts, which is fundamental for minimizing the risk of spread. In addition, the diagnosis, isolation, and management of the asymptomatic cases are also highlighted. 34 Thus, more studies and efforts are needed to prevent the spread of SARS‐CoV‐2.
For the discharged patients, according to the diagnostic and treatment guidelines for COVID‐19, issued by the Chinese National Health Committee (version 6‐7), the designated hospitals should contact the primary health care facilities where the patients reside, and patients' medical records should be shared after the discharge. It is recommended for patients to live in a single, well‐ventilated room, if possible. Also, returning to the hospitals for follow‐up in 2 and 4 weeks after the discharge is also recommended.
To sum up, the COVID‐19 infection has a clustering onset. It can infect people of all ages. Older age, more comorbidities, and more abnormal prominent laboratory markers were associated with a more severe conditions. Effective diagnosis and timely treatment programs can effectively reduce the severity of patients' conditions and significantly reduce patients' mortality. More efforts should be made to better understand the whole spectrum and pathophysiology of COVID‐19.
SUMMARY
SARS‐CoV‐2 can infect people of all ages, the majority of whom suffer from abnormal body temperature, and chest CT imaging, early identification, and timely medical treatment are of significant importance to reduce the severity of patients with COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LS, HF, JB, and LS conceived and designed the study. LS, LS, FG, MH, YA, and QZ collected the data. HF, LS, LS, and JF analyzed the data. LS, HF, and JF wrote the paper. This study has been read and approved by all of the authors. The requirements for authorship, as stated earlier in this document, have been met, and each author believes that the manuscript represents an honest work.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by grants from the National Key Research and Development Plan of China (2018ZX09711003‐004‐003), Fundamental Research Funds for Central Universities (BUCTZY2012), and China Postdoctoral Science Foundation (2019M65072).
Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol. 2020;92:2055–2066. 10.1002/jmv.25966
Lijun Sun, Lijun Shen, and Junfen Fan contributed equally to this study.
Contributor Information
Huahao Fan, Email: fanhuahao@mail.buct.edu.cn.
Jingfeng Bi, Email: 123bjf@163.com.
REFERENCES
- 1. Wu J, Liang J, Zhou H, et al. Clinical features and outcomes of asymptomatic cases of SARS‐CoV‐2 infection. J Infect. 2020. 10.1016/j.jinf.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Health Commission and National Administration of Traditional Chinese Medicine . Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl). 2020;133:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Geneva, Switzerland: World Health Organization. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 7. Lin L, Li TS. Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019‐nCoV) Infection by the National Health Commission (Trial Version 5)". Zhonghua Yi Xue Za Zhi. 2020;100:E001. [DOI] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishiura H, Linton NM, Akhmetzhanov AR. Initial cluster of novel coronavirus (2019‐nCoV) infections in Wuhan, China is consistent with substantial human‐to‐human transmission. J Clin Med. 2020;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu P, Zhu J, Zhang Z, Han Y, Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person‐to‐person transmission during the incubation period. J Infect Dis. 2020;221:1757–1761. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu WJ, Zhao M, Liu K, et al. T‐cell immunity of SARS‐CoV: implications for vaccine development against MERS‐CoV. Antiviral Res. 2017;137:82‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 18. Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787–1799. 10.1056/nejmoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa‐2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977‐1985. [DOI] [PubMed] [Google Scholar]
- 22. Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020:64. 10.1128/aac.00399-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19). JAMA. 2020. 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 24. Sheahan TP, Sims AC, Graham RL, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. 10.1126/scitranslmed.aal3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Touret F, de Lamballerie X. Of chloroquine and COVID‐19. Antiviral Res. 2020;177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019‐novel coronavirus (2019‐nCoV) related coronavirus model. Chin Med J (Engl). 2020;133:1051‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 30. Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA‐dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773‐4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS‐Coronavirus‐2 in Shenzhen, China. J Infect Dis. 2020;221(11):1770–1774. 10.1093/infdis/jiaa119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


