Abstract
There are currently no proven or approved treatments for coronavirus disease 2019 (COVID‐19). Early anecdotal reports and limited in vitro data led to the significant uptake of hydroxychloroquine (HCQ), and to lesser extent chloroquine (CQ), for many patients with this disease. As an increasing number of patients with COVID‐19 are treated with these agents and more evidence accumulates, there continues to be no high‐quality clinical data showing a clear benefit of these agents for this disease. Moreover, these agents have the potential to cause harm, including a broad range of adverse events including serious cardiac side effects when combined with other agents. In addition, the known and potent immunomodulatory effects of these agents which support their use in the treatment of auto‐immune conditions, and provided a component in the original rationale for their use in patients with COVID‐19, may, in fact, undermine their utility in the context of the treatment of this respiratory viral infection. Specifically, the impact of HCQ on cytokine production and suppression of antigen presentation may have immunologic consequences that hamper innate and adaptive antiviral immune responses for patients with COVID‐19. Similarly, the reported in vitro inhibition of viral proliferation is largely derived from the blockade of viral fusion that initiates infection rather than the direct inhibition of viral replication as seen with nucleoside/tide analogs in other viral infections. Given these facts and the growing uncertainty about these agents for the treatment of COVID‐19, it is clear that at the very least thoughtful planning and data collection from randomized clinical trials are needed to understand what if any role these agents may have in this disease. In this article, we review the datasets that support or detract from the use of these agents for the treatment of COVID‐19 and render a data informed opinion that they should only be used with caution and in the context of carefully thought out clinical trials, or on a case‐by‐case basis after rigorous consideration of the risks and benefits of this therapeutic approach.
Keywords: coronavirus, COV‐SARS‐2, immunology, immune, SARS
Abbreviations
- ACE‐2
angiotensin‐converting enzyme 2
- ARDS
acute respiratory distress syndrome
- BID
bis in die (twice per day)
- COVID‐19
coronavirus disease 2019
- CQ
chloroquine
- CRP
C reactive protein
- ECG
electrocardiogram
- HCQ
hydroxychloroquine
- ICU
intensive care unit
- IVIG
intravenous immunoglobulin
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- PCR
polymerase chain reaction
- RT‐PCR
reverse transcription‐polymerase chain reaction
- SARS‐CoV
severe acute respiratory syndrome coronavirus
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has caused a global pandemic that is severely straining health systems everywhere. 1 COVID‐19 has an estimated symptomatic case fatality rate of approximately 1.4% which is around 15 times greater than that estimated for seasonal influenza. 2 , 3 , 4 The mortality rate rises dramatically for individuals with increasing age and comorbidities. 5
There are currently no proven or approved treatments for this disease, though numerous therapeutic agents are under investigation. The illness course is variable,: some individuals are asymptomatic, others experience a mild, self‐resolving flu‐like illness, and others still progress to moderate or severe disease. 6 For those who progress to more severe disease, there are typically four phases of the illness course, see Figure 1. The first is the incubation period which lasts a median of 5.1 days, with a large range. 7 The second is a mild symptomatic phase which lasts around 5 days and typically includes flu‐like symptoms including fever, cough, myalgias, and fatigue, though gastrointestinal symptoms like anorexia, nausea, vomiting, and diarrhea as well as anosmia can be prominent. 1 , 6 This is followed by progression to a hyperinflammatory acute respiratory distress syndrome (ARDS). 8 The onset of this third phase is typically marked by dyspnea, tachypnea, and progressive, sometimes silent hypoxemia. This phase is marked by high fevers, elevated inflammatory markers, and the progressive formation of bilateral diffuse pulmonary opacities on chest radiographs and associated respiratory failure. Some individuals develop multisystem organ failure with complications that can include micro and macro thromboses, myocarditis, elevated muscle enzymes suggestive of myositis, and kidney failure. 9 , 10 , 11
Figure 1.
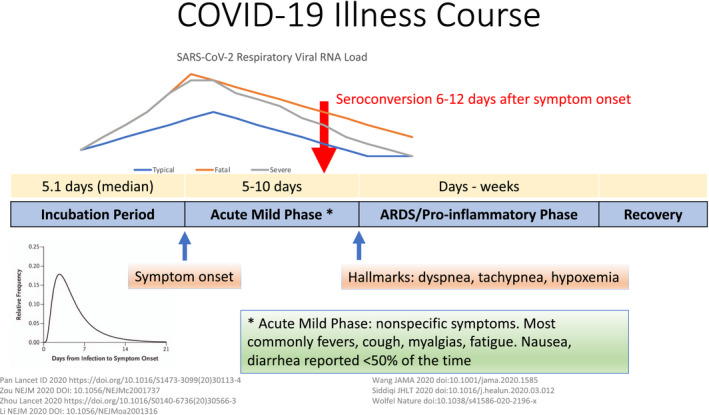
COVID‐19 clinical course of illness. The first phase of COVID‐19 infection involves an incubation period of variable duration, with a median of 5.1 days. The second is an acute mild phase that most commonly includes flu‐like symptoms like cough, fevers, and myalgias, but can also include gastrointestinal symptoms. Some patients progress to an ARDS hyperinflammatory phase that is often marked by dyspnea, tachypnea, and hypoxemia. The respiratory viral load rises before the onset of symptoms and peaks around the onset of symptoms. It declines over the first week. Severe cases have higher viral loads compared with mild cases. Prolonged viral shedding in severe and mild cases is reported
Because of the severity of the illness course in some cases of COVID‐19, effective treatments are desperately needed. Unfortunately, few high quality randomized controlled treatment trials have been published to date for investigational agents for this disease. To date, hydroxychloroquine (HCQ) and chloroquine (CQ) have been widely used around the world for COVID‐19 and previously for Ebola, H7N9 influenza and SARS virus infection, based on very limited data, though they remain unproven and of unknown benefit. The efficacy of HCQ may depend on the timing of administration, as it is being considered for post‐exposure prophylaxis and all stages of infection. Additionally, certain safety concerns have been raised about these agents, not only because of their long half‐life but also because of their potential adverse effects when combined with certain other drugs. Here we will review the known effects of HCQ on virus replication and the immune system and present the evidence to date and notable considerations for HCQ therapy in patients with COVID‐19.
2. MECHANISM OF ACTION
HCQ and CQ have been used for many years to treat a number of diseases, including auto‐immune diseases like lupus and rheumatoid arthritis as well as for the prevention and treatment of malaria. 12 These agents are known to raise intracellular pH and, in particular, affect endosomal activity. 13 , 14 This action has wide‐ranging secondary effects, including potent immune modulation via specific mechanisms. In the context of COVID‐19, limited data exist on the antiviral activity of these agents. Both antiviral and immune modulatory activities are discussed below, as are considerations for potential adverse reactions and drug interactions.
3. PRE‐CLINICAL DATA AND DATA FOR THE ANTIVIRAL ACTIVITY OF CQ AND HCQ INCLUDING AGAINST SARS‐LIKE CORONAVIRUSES
Following the emergence of SARS, CQ was found to be a potent in vitro inhibitor of SARS‐CoV. Keyaerts and colleagues showed that no significant viral replication was measured in Vero E6 cells inoculated with SARS‐CoV in the presence of CQ. 15 The authors did note that antiviral activity diminished as CQ was added later to the cells, suggesting that the mechanism of action may be earlier in the viral life cycle. Later, Vincent et al showed that CQ when added post‐infection to Vero E6 cells could markedly decrease the number of infected cells. 16 They suggested that the mechanism of action might include the elevation of endosomal pH, disruption of intracellular transport of the virus, as well as altered glycosylation of angiotensin‐converting enzyme 2 (ACE‐2) potentially reducing SARS‐CoV‐2 binding to ACE‐2 and therefore preventing viral entry. Though it is unclear how CQ and HCQ may impact SARS‐CoV‐2 engagement with other surface‐exposed proteins reported to interact with SARS‐CoV and SARS‐COV‐2. 17 , 18 , 19 , 20
Similar work was performed after the emergence of a related virus called the Middle East respiratory syndrome coronavirus (MERS‐CoV). While CQ was shown to have anti‐MERS activity in immortalized cell lines, later studies showed that MERS‐CoV can rapidly infect certain antigen‐presenting cells and that CQ did not inhibit the infection of or viral replication in these cells. 21 , 22
After the emergence of SARS‐CoV‐2, scientists explored drugs that might have efficacy against this novel virus. CQ was rapidly identified as having potent activity in vitro against the virus. 23 The mechanism of action was thought to be the same as was proposed for its activity against SARS‐CoV. However, there are no published series or randomized clinical trials of patients with SARS‐CoV or MERS‐CoV treated with HCQ or CQ.
4. PHARMACOLOGY AND SAFETY
HCQ has been used for a long time in diseases such as rheumatoid arthritis, systemic lupus erythematosus, and malaria. As noted previously, CQ and HCQ are efficiently absorbed, reach peak serum concentrations in 2‐3.5 hours, have an elimination half‐life of 22 to 45 days, and can reach serum concentrations of approximately 1.5 µm through the administration of 6.5 mg/kg/day. 12 , 24 , 25 , 26 , 27 , 28 It is important to note that discrepancies in concentrations and half‐life measurements can result from varied methods and source material for estimation, and that measurement in whole blood is preferred over serum or plasma. 29 , 30 Administration of CQ and HCQ in animal models demonstrated splenic, renal, hepatic, and importantly lung accumulation levels 200‐700 times greater than in plasma, and more recent studies also noted that melanin binding contributes to skin and eye accumulation. 31 , 32 There are several well‐described side effects with long‐term use, such as cardiomyopathy and retinal toxicity. While short‐term use of HCQ has a substantially lower risk of cardiomyopathy and retinopathy, there remain concerns related to QT prolongation, hypoglycemia, as well as side effects such as gastrointestinal disturbance.
Given the increase in the use of HCQ for COVID‐19, a group recently published their analysis of the risk for adverse events associated with this medication. 33 They included more than 950 000 HCQ users of whom more than 320 000 had combination therapy with azithromycin. They found no elevated risk for adverse events for short term HCQ treatment (defined as within the first 30‐days after starting therapy) compared with equivalent therapy for individuals with rheumatoid arthritis treated with sulfasalazine. However, they did find a 15%‐20% increased risk of chest pain or heart failure and a twofold increased risk of cardiovascular mortality in the first month of treatment following the addition of azithromycin to HCQ. This finding highlights the concern that the combination of these two medications may place patients at increased health risk. Given the lack of evidence that HCQ is beneficial for the treatment of COVID‐19, these two agents should likely not be combined for COVID‐19 outside of a clinical trial unless there are strong indications for each, no viable alternatives in individual patients and they are administered with appropriate counseling and close monitoring for adverse events.
5. CLINICAL DATA FOR HCQ AND CQ IN COVID‐19
Based on the initial in vitro data, there was early clinical interest in using CQ and HCQ for the treatment of COVID‐19. A group from China reported an interim analysis of outcomes for more than 100 patients with COVID‐19 treated with CQ and noted it was superior to the control. There were, unfortunately, no details about these patients in the report, though they reported that patients who received CQ had less severe pneumonia, improved lung imaging, earlier conversion of viral shedding, and a shorter disease course. 34 They also reported that this treatment was safe. This led to the release of an expert consensus in China that recommended high dose CQ phosphate at 500 mg twice per day (BID) for 10 days for patients with mild, moderate or severe COVID‐19. 35
Since that time, several small studies have been released looking at HCQ and CQ in more detail. The first was a small randomized control trial of 30 patients with COVID‐19 in China who were randomized 1:1 to receive HCQ or not, in addition to other antivirals that included interferon alpha, umifenovir, and lopinavir/ritonavir. 36 They found no difference in radiographic progress, time to becoming afebrile, or percent who had a negative throat swab at day 7. Shortly thereafter a small non‐randomized clinical trial was released from France for hospitalized non‐intensive care unit (ICU) patients which reported HCQ led to dramatically faster viral clearance from nasopharynx compared with control. 37 They reported that nearly 60% of patients on HCQ had day 6 viral clearance compared with under 15% of controls. In this methodologically flawed study, 26 patients were in the HCQ arm and 16 in the control arm. The analysis excluded six patients from the analysis who tended to be sicker. The study has since been widely criticized, and the society that published the study subsequently released a statement saying it did not meet their standards. 38
This same French group reported that among the six patients who received HCQ and azithromycin, viral clearance from nasopharynx was fastest; they suggested this might indicate synergy between the two agents. 37 They subsequently released another report of a non‐randomized series of 80 patients with mild COVID who had been treated with HCQ and azithromycin. 39 Overall, the patients were admitted with an average of 5 days of symptoms, 93% had a day 8 negative reverse transcription‐polymerase chain reaction (RT‐PCR) from the nasopharynx, and 81.3% were discharged at the time of writing the report with an average hospital length of stay of just over 4.5 days. They had no comparison arm in this second report.
A second French group studied the combination of HCQ and azithromycin in 11 patients with COVID‐19. 40 They found that in the 10 patients who were alive at day 6, only two had viral clearance, casting further doubt on the initial report from the first French group, where reported viral detection rates at day 6 were so much lower.
Another small randomized controlled trial from China looked at HCQ vs no HCQ for mild COVID. 41 They reported that the resolution of cough and fever was faster in the HCQ arm. However, there were some important limitations to the study. First, 80 patients were excluded from the study for unclear reasons. Second, the standard therapies that were allowed in each group included steroids, antivirals, and intravenous immunoglobulin (IVIG) but how these were distributed between the groups was not reported. There are no data about discharge, mortality, and viral clearance and therefore the endpoints they reported may not be meaningful.
In a preprint of a retrospective analysis of 181 hospitalized patients with COVID‐19 in France, authors compared 84 patients who received HCQ (600 mg/day) within 48 hours of admission to 97 patients who did not. 42 The baseline characteristics were similar between the groups. The patients included had symptoms for a median of 7 days before admission to the hospital. They were enrolled if they were admitted to the hospital but not in the intensive care unit and were on ≥2 L of supplemental oxygen. Although this was not a randomized trial, no benefit was found for the group that received HCQ and approximately 10% who received this medication needed it discontinued because of changes in their electrocardiograms (ECGs).
A further recent preprint from China reports the largest randomized controlled trial studying HCQ to date. 43 It included 150 hospitalized patients with COVID‐19 and randomized them 1:1 to receive HCQ plus standard of care or standard of care in an open‐labeled fashion. The vast majority of the patients had mild to moderate disease (99%) and the mean day from symptom onset to randomization was 16.6 days. HCQ was dosed as a loading dose of 1200 mg daily for 3 days followed by a maintenance dose of 800 mg daily for 2‐3 weeks, with longer courses given for severe patients. The primary endpoint was conversion to negative SARS‐CoV‐2 polymerase chain reaction (PCR) from upper and/or lower respiratory tract samples by day 28. They found no difference in negative SARS‐CoV‐2 conversion by day 28 for each group with 85.4% negative in the HCQ arm and 81.3% negative in the standard of care arm, P = .341. Negative conversion rates were also similar at earlier time points. In the initial analysis there was no difference in symptom improvement by day 28; however, in a post hoc analysis controlling for confounding effects of other antivirals suggested improvement in symptoms in the HCQ group compared with the control group and the HCQ group did have a greater reduction in C reactive protein (CRP). The primary outcome for this trial was negative, and overall the trial did not support the use of HCQ late in the course of COVID‐19. For a summary of all of the human data for HCQ and CQ for COVID‐19, please see Table 1.
Table 1.
Summary of human studies with HCQ/CQ for COVID‐19 to date
| References | RCT? | Total population in the study | Outcome | Notes |
|---|---|---|---|---|
| 34 | No | >100 | Report of superiority of CQ | No details about patients in the study |
| 35 | No | N/A | N/A | Expert consensus recommending CQ for all with COVID‐19 in China |
| 36 | Yes | 30 | No difference | HCQ + standard of care (SOC) vs SOC |
| 37 | No | 42 | Report faster viral clearance with HCQ | Publisher has since said that the report did not meet their standards |
| 39 | No | 80 | No comparison group | |
| 40 | No | 11 | No evidence of fast viral clearance with HCQ | Casts further doubt on reports from Gautret et al |
| 41 | Yes | 62 | Faster resolution of cough and fever with HCQ | Not clear these endpoints matter |
| 42 | No | 181 | No benefit to HCQ | |
| 43 | Yes | 150 | Did not meet primary outcome, but CRP declined faster with HCQ | HCQ started late in disease course (mean 16.6 days after onset) |
| 44 | Yes | ~80 | Stopped early because high dose CQ led to more adverse events |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Finally, in a recent study in Brazil participants were randomized to receive high dose CQ (600 mg BID for 10 days) vs low dose CQ (450 BID × 1 day then 450 mg daily × 4 days). 44 Additionally, all patients were treated with ceftriaxone and azithromycin. More than 25% of patients in the high dose arm developed a prolonged QTc > 500 ms. The high dose group had an increased mortality rate of 17% vs 13.5%. There was no evidence for the rapid clearance of viral load on their testing. Because of concerns about safety and no clear benefit to the higher dose of CQ the study was stopped.
6. HCQ AND OTHER VIRAL INFECTIONS
It is worth noting that HCQ and CQ have in vitro activity against a number of viruses, including influenza, but they have yet to show clinical benefit for viral infections. 45 In fact, a large clinical trial in Singapore sought to determine if HCQ could reduce influenza infection among 1500 patients randomized 1:1 who received CQ (500 mg/day for 1 week then once a week for 12 weeks) or matching placebo, monitoring for symptoms and laboratory evidence of influenza in each group. 46 There was no difference in the incidence of influenza and more patients in the CQ group reported adverse events (45% vs 33%) including headache, dizziness, nausea, and diarrhea most commonly.
7. HCQ AND THE IMMUNE SYSTEM
The primary use of HCQ, beyond its well‐established role as an antimalarial agent, is as an immunomodulator for autoimmune diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In this context, HCQ disrupts lysosomal antigen processing by antigen‐presenting cells and lowers T‐cell recruitment and subsequent production of pro‐inflammatory cytokines including IL‐6 and TNFα. 47 While the ultimate effect of this modulation of the immune system is favorable for treating auto‐immune conditions, it is not clear how these effects of HCQ may affect a person with acute COVID‐19. It is important to note that as observed with SARS, patients with COVID‐19 can exhibit leuko‐ and lymphopenia, that NK and CD8 T‐cells exhibit markers (NKG2A, PD‐1) and diminished function consistent with exhaustion. 48 , 49 , 50 Further, the extent of functional exhaustion and reduced diversity may correlate with the risk of severe disease, while restoration of monocytes and lymphocytes is linked with improved viral clearance and recovery. 51 , 52 , 53 Key points to consider are summarized in Figure 2 and Table 2 and discussed in further detail below.
Figure 2.
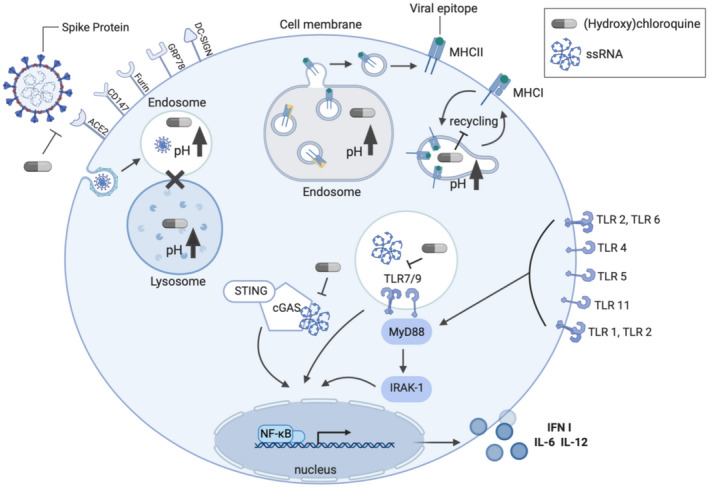
Schematic—proposed mechanisms of action of HCQ in SARS‐CoV‐2 infection. HCQ can limit coronavirus infection and reduce inflammatory and immune cell function. Treatment with HCQ alters the n‐terminal glycosylation of ACE‐2, which can reduce the affinity of ACE2‐S1 (Spike) interactions, though the impact on the interaction of other relevant surface proteins is unclear. HCQ can also inhibit viral infection by disrupting endosomal acidification to interfere with viral fusion. Induction of cytokine expression resulting from innate immune signaling is also impacted by HCQ mediated reduction in DNA/RNA binding and activation of cGAS/STING signaling and altered endosomal pH also disrupts binding to TLR7/9. Elevated endosomal pH can also alter (cross‐)presentation of antigen by MHC Class I and II, modifying the development and activation of antigen‐specific T cell and B cell populations
Table 2.
Impact of (hydroxy)chloroquine on major immune populations
| Immune cell | Antiviral activity | Impact of (hydroxy)CQ |
|---|---|---|
| Plasmacytoid dendritic cells (pDC) | In response to viral infection pDCs are activated and produce high levels of IFN‐I. Activated pDCs induce the activation of the adaptive immune response | Inhibits pDC maturation and IFN‐I production |
| Macrophages | Activated through TLR3 binding of dsRNA, promoting macrophage secretion of pro‐inflammatory cytokines | Reduces TNF‐α, IL‐1β and IL‐6 synthesis |
| Natural Killer cells | NK cells produce IFN‐γ and TNFα in response to a viral infection. NK‐cells recognize low MHC‐I presentation on virus‐infected cells and release perforin causing lysis of the target cell | Inhibiting the processing of perforin to its active form, consequently reducing NK cell cytotoxicity |
| CD4 T cells | Upon activation produce IFN‐γ and IL‐4. Regulates B‐ lymphocyte and CTL antiviral responses | Downregulated antigen presentation by MHC, limiting the stimulation of CD4 T‐cells and its expression of CD154 |
| CD8 T cells | Upon activation present cytotoxic activity against viral‐infected cells | Inhibits cytotoxic activity by inhibiting lysosomal release |
| B Cells | Production of virus‐specific antibodies | Altered endosomal pH modulates antigen presentation, biases selection of naïve antigen‐specific B cells, reducing affinity maturation of previously engaged clones |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
While it is a potent immune modulator, HCQ is not considered immunosuppressive and has not to date been associated with elevated infection risk. 54 In the context of its long‐term use for rheumatologic disease, HCQ has also not been associated with an increased the risk of infection. 55 A large study of more than 16 000 patients with rheumatoid arthritis (RA) found no increased risk for pneumonia for those on this agent. 54 Similarly, another cohort of more than 23 000 patients with RA found no elevated risk for infection requiring hospitalization for those on HCQ compared with other agents. 55 A third large cohort study of more than 24 000 patients with RA also found the risk of infection requiring hospitalization or outpatient parenteral antibiotics was less for those on HCQ compared with biologic disease‐modifying antirheumatic drugs. 56 Additionally, a small case‐control study of patients with SLE matched 65 patients that developed herpes zoster with 130 that did not and found a reduced the risk of Herpes zoster infection in those receiving HCQ. 57 Furthermore, there has been no signal of elevated infection risk or exacerbation of chronic infections for patients on HCQ and there is, therefore, no warning about infection risk on the FDA label on this drug.
HCQ affects and modulates the innate immune system and response to viral infections. In the context of SARS‐CoV‐2 infection, disruption of vesicle acidification by HCQ is postulated to have antiviral effects and reduce the overabundant production of cytokines. However, dysregulation of vesicular acidification by HCQ may have additional effects on innate and adaptive immune responses of patients with COVID‐19 with unknown consequences. The typical innate immune response to SARS‐CoV‐2 is marked by suppressed type I interferon. TLR 7 plays an important role in the recognition of SARS‐CoV‐2 RNA and initiating the host innate immune response. 58 By increasing endosomal pH, HCQ reduces TLR 7 and TLR 9 affinity for viral RNA, thereby diminishing the induction of cytokines which may play a role in viral control including type I interferon, IL‐6, and IL‐12. It is important to note that MyD88‐dependent signaling is not impacted by CQ, though it can be modulated by SARS‐CoV and is necessary for the protection in a murine challenge model. 59 Furthermore, HCQ inhibits cyclic GMP‐AMP synthase (cGAS) activity in host cells. 60 Activation of the cGAS/STING pathway by RNA/DNA‐dependent mechanism promotes increased type I interferon (IFNβ) production. It is known that SARS‐CoV induces cGAS/STING and that SARS‐CoV‐2 is highly responsive to type I interferon. 61 , 62 Additionally, HCQ reduces NK cell cytotoxic function by limiting perforin processing to its functional form. 63 Relevant to reducing cytokine levels, a study of patients receiving CQ for Early Persistent Musculoskeletal Pain and Arthritis who were also infected with Chikungunya Virus demonstrated that IL‐6 and IL‐13 levels remained strongly up‐regulated throughout the study period. 64 The ultimate effects of HCQ on the innate immune response to SARS‐CoV‐2 remain unknown but are clearly important to consider in this clinical context.
In addition to attenuating innate immune signaling, HCQ also impacts the adaptive immune response. HCQ inhibition of endosome acidification also affects antigen processing and presentation, which, in turn, alters both T‐cell and B‐cell responses. Treatment with CQ/HCQ reduces the number of rapidly proliferating T‐cells and limits differentiation toward Th1 and Th17. 65 , 66 Reduced antigen presentation limits the induction of CD4 helper T‐cells, specifically the expression of CD154, reducing IL‐6 and TNFα production. 67 In vitro inhibition of autophagy with CQ during T‐cell activation rendered T helper cells hypo‐responsive to re‐stimulation with the antigen, reduced proliferation, and less IL‐2 production. 68 In CD8 T‐cells, HCQ limits degranulation and adversely impacts cytotoxic function in vitro, it though does increase cross‐presentation and IFNγ production in memory CD8 cells re‐stimulated with antigen (both in vitro and in vivo in humans). 69 , 70 , 71 , 72
In a study of influenza vaccination in patients with SLE, HCQ did not adversely impact seroconversion. 73 However, it is important to note that HCQ does bias selection of antigen‐specific B‐cells toward naïve cells and away from affinity maturation, thereby reducing clonal expansion which may have important implications for the generation of neutralizing antibodies. 74 , 75 This is particularly significant due to concerns related to antibody‐dependent enhancement (ADE) of viral infection. ADE occurs when non‐neutralizing antibodies bind the virus, thereby promoting entry through the FC‐receptor, and is of particular concern when patients exhibit low levels of neutralizing antibodies. 76 Furthermore, though long‐term administration does not appear to negatively impact vaccination to prevent bacterial and viral infections, antibody‐mediated responses are diminished by concurrent vaccination and short‐course treatment with HCQ. 77 , 78 , 79 , 80 Taken together, these observations highlight that the immunomodulatory effect of HCQ on COVID‐19 remains unknown. HCQ may contribute to dampening an overly exuberant immune response during the inflammatory phase of the infection and enhance cross‐presentation to CD8 T‐cells and their IFNγ production. It is also possible that acute treatment with HCQ may weaken the innate immune response to the virus, impair adaptive immune responses, and could, in the worst case, alter the repertoire of T‐cells and B‐cells generated in response to SARS‐CoV‐2, potentially reducing the efficacy of recall responses to re‐exposure or even put them at risk for antibody‐mediated enhancement. The activity of HCQ in the context of virus‐induced inflammation, innate immune activity, and nascent adaptive responses creates a complex milieu that will require careful study to decipher and discern which patients, dosing, and stage of the disease may benefit from this intervention.
8. DISCUSSION
As hospitals around the globe have filled with patients with COVID‐19, front line providers remain without effective therapeutic tools to directly combat the disease. The initial anecdotal reports out of China led to the initial wide uptake of HCQ and to a lesser extent CQ for many hospitalized patients with COVID‐19 around the globe. As more data have become available, enthusiasm for these medications has been tempered. Well designed, large randomized controlled trials are needed to help determine what role, if any, these medications should have in treating COVID‐19 moving forwards.
While HCQ has in vitro activity against a number of viruses, it does not act like more typical nucleoside/tide antiviral drugs. For instance, HCQ is not thought to act on the critical viral enzymes including the RNA‐dependent RNA polymerase, helicase, or proteases. Despite in vitro activity against influenza, in a large high quality randomized controlled trial, it showed no clinical benefit, suggesting that similar discordance between in vitro and in vivo observations is possible for SARS‐CoV and SARS‐CoV‐2 73 (Table 3).
Table 3.
Comparison of reported in vitro inhibition of influenza and COV‐SARS, COV‐SARS‐2
| Treatment | CQ | HCQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Virus | H1N1 | H3N2 | SARS‐CoV‐1 | SARS‐CoV‐1 | SARS‐CoV‐2 | SARS‐CoV‐2 | SARS‐CoV‐2 | SARS‐CoV‐2 |
| IC50/EC50 (μm) | 3.6 | 0.84 | 4.4 | 8.8 | 5.47 | 2.71 | 0.72 | 4.51 |
| Incubation | 16‐18 hr | 16‐18 hr | 16‐18 hr | 3 days | 48 hr | 1 hr pre + 48 hr | 48 hr | 1 hr pre + 48 hr |
| Citations | 81 | 81 | 16 | 15 | 82 | 25 | 82 | 25 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Additionally, HCQ and especially CQ have cardiovascular and other risks, particularly when these agents are used at high doses or combined with certain other agents. While large scale studies have demonstrated that long‐term treatment with CQ or HCQ does not increase the incidence of infection, caution should be exercised in extrapolating safety from the studies of chronic administration to largely healthy individuals to estimate the risk associated with short‐course treatment in acutely and severely ill patients. Furthermore, the immunologic actions that make HCQ an important drug for the treatment of auto‐immune diseases might have unintended consequences when it is used for patients with COVID‐19. The effects of this immune modulation on patients with COVID‐19 are unknown at this time, including a potential negative impact on antiviral innate and adaptive immune responses which need to be considered and studied.
For all these reasons, and in the context of accumulating preclinical and clinical data, we recommend that HCQ only be used for COVID‐19 in the context of a carefully constructed randomized clinical trial. If this agent is used outside of a clinical trial, the risks and benefits should be rigorously weighed on a case‐by‐case basis and reviewed in light of both the immune dysfunction induced by the virus and known antiviral and immune modulatory actions of HCQ.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.A. Meyerowitz, P.M. Reeves, and M.C. Poznansky designed the review; E.A. Meyerowitz, P.M. Reeves, J.A. Gelfand, A.G.L. Vannier, M.G.N. Friesen, M.V. Callahan, S. Schoenfeld, A.Y. Kim, M.C. Poznansky wrote, and reviewed the manuscript and performed a detailed literature research; E.A. Meyerowitz, P.M. Reeves, A.G.L. Vannier, M.G.N. Friesen, and M.C. Poznansky performed the literature research; E.A. Meyerowitz, P.M. Reeves, A.G.L. Vannier, M.G.N. Friesen, assembled the tables; E.A. Meyerowitz created the illustration in Figure 1; M.G.N. Friesen created the illustration in Figure 2.
ACKNOWLEDGMENTS
The authors thank the patients and their families suffering from COVID‐19 infection and the healthcare workers including doctors and nurses involved in their care at Massachusetts General Hospital.
Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID‐19. The FASEB Journal. 2020;34:6027–6037. 10.1096/fj.202000919
Augustin G. L. Vannier and Morgan G. N. Friesen contributed equally to this manuscript.
This article was fast‐tracked under a recently instituted interim policy in which editors may, at their discretion, accept coronavirus‐related manuscripts submitted for the Review, Perspectives, and Hypotheses categories without additional review.
The views of Michael V. Callahan expressed here do not reflect an official position of the U.S. Department of Health and Human Services.
The views of Michael V. Callahan expressed here do not reflect an official position of the U.S. Department of Health and Human Services.
REFERENCES
- 1. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID‐19 from the transmission dynamics in Wuhan, China. Nat Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect. Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizumoto K, Chowell G. Estimating risk for death from 2019 novel coronavirus disease, China, January‐February 2020. Emerg Infect Dis. 2020:26(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states. A clinical‐therapeutic staging proposal. J. Hear Lung Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rainsford KD, Parke AL, Clifford‐Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231‐269. [DOI] [PubMed] [Google Scholar]
- 13. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Trani L, Savarino A, Campitelli L, et al. Different pH requirements are associated with divergent inhibitory effects of chloroquine on human and avian influenza A viruses. Virol J. 2007;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fung TS, Liu DX. Human coronavirus: host‐pathogen interaction. Annu Rev Microbiol. 2019;73:529‐557. [DOI] [PubMed] [Google Scholar]
- 18. Wang K, Chen W, Zhou Y‐S, et al. SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv. 2020. [Google Scholar]
- 19. Aguiar JA, Tremblay BJ‐M, Mansfield MJ, et al. Gene expression and in situ protein profiling of candidate SARS‐CoV‐2 receptors in human airway epithelial cells and lung tissue. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID‐19 spike‐host cell receptor GRP78 binding site prediction. J Infect. 2020;80(5):554‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cong Y, Hart BJ, Gross R, et al. MERS‐CoV pathogenesis and antiviral efficacy of licensed drugs in human monocyte‐derived antigen‐presenting cells. PLoS ONE. 2018;13:e0194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA‐approved compound library identifies four small‐molecule inhibitors of middle east respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875‐4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laaksonen AL, Koskiahde V, Juva K. Dosage of antimalarial drugs for children with juvenile rheumatoid arthritis and systemic lupus erythematosus. A clinical study with determination of serum concentrations of chloroquine and hydroxychloroquine. Scand J Rheumatol. 1974;3:103‐108. [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27:771‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim HS, Im JS, Cho JY, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. Antimicrob Agents Chemother. 2009;53:1468‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. FDA/CDER . Plaquenil ® Hydroxychloroquine Sulfate Tablets, Usp Description. White Oak, MD: FDA; 2017. [Google Scholar]
- 29. Munster T, Gibbs JP, Shen D, et al. Hydroxychloroquine concentration‐response relationships in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1460‐1469. [DOI] [PubMed] [Google Scholar]
- 30. Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheumatol. 2015;42:2092‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Popert AJ. Chloroquine: a review. Rheumatol Rehabil. 1976;15:235‐238. [DOI] [PubMed] [Google Scholar]
- 32. Collins KP, Jackson KM, Gustafson DL. Hydroxychloroquine: a physiologically‐based pharmacokinetic model in the context of cancer‐related autophagy modulation. J Pharmacol Exp Ther. 2018;365:447‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lane JCE, Weaver J, Kostka K, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide‐spread use for COVID‐19: a multinational, network cohort and self‐controlled case series study. medRxiv. 2020. [Google Scholar]
- 34. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72‐73. [DOI] [PubMed] [Google Scholar]
- 35. Multicenter Collaboration Group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for Chloroquine in the Treatment of Novel Coronavirus Pneumonia . Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. [DOI] [PubMed] [Google Scholar]
- 36. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). J Zhejiang Univ (Med Sci). 2020;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrobial Agent. 2020;105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Voss A. ,(ISAC). Official Statement from International Society of Antimicrobial Chemotherapy (ISAC) on: Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial (Gautret P et al. PMID 32205204). Statement IJAA Pap. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Gautret P, Lagier J‐C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med. Infect. Dis. 2020:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Méd Mal Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv. 2020. [Google Scholar]
- 42. Mahevas M, Tran V, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID‐19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv. 2020:1‐20. [Google Scholar]
- 43. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID‐19: an open‐label, randomized, controlled trial. medRxiv. 2020. [Google Scholar]
- 44. Borba MGS, Val FFA, Sampaio VS, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS‐CoV‐2) infection: Preliminary safety results of a randomized, double‐blinded, phase IIb cl. medRxiv. 2020. [Google Scholar]
- 45. Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID‐19 pandemic: what every clinician should know. Ann Intern Med. 2020:19‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paton NI, Lee L, Xu Y, et al. Chloroquine for influenza prevention: a randomised, double‐blind, placebo controlled trial. Lancet Infect Dis. 2011;11:677‐683. [DOI] [PubMed] [Google Scholar]
- 47. Jang CH, Choi JH, Byun MS, Jue DM. Chloroquine inhibits production of TNF‐alpha, IL‐1beta and IL‐6 from lipopolysaccharide‐stimulated human monocytes/macrophages by different modes. Rheumatol. 2006;45:703‐710. [DOI] [PubMed] [Google Scholar]
- 48. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tu Y, Chien C, Yarmishyn AA, et al. A review of SARS‐CoV‐2 and the ongoing clinical trials. Int J Mol Sci. 2020;21:2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non‐severe COVID‐19. Nat Med. 2020;26:453‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen X, Ling J, Mo P, et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID‐19 hospitalized patients. medRxiv. 2020. [Google Scholar]
- 54. Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease‐modifying antirheumatic drugs, and anti‐tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628‐634. [DOI] [PubMed] [Google Scholar]
- 55. Bernatsky S, Hudson M, Suissa S. Anti‐rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology. 2007;46:1157‐1160. [DOI] [PubMed] [Google Scholar]
- 56. Smitten AL, Choi HK, Hochberg MC, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35:387‐393. [PubMed] [Google Scholar]
- 57. Zamora LD, Collante MTM, Navarra SV. Risk factors for herpes zoster infection among Filipinos with systemic lupus erythematosus. Int J Rheum Dis. 2020;23:197‐202. [DOI] [PubMed] [Google Scholar]
- 58. Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sheahan T, Morrison TE, Funkhouser W, et al. MyD88 is required for protection from lethal infection with a mouse‐adapted SARS‐CoV. PLoS Pathog. 2008;4:e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: antimalarial drugs inhibit IFN‐beta production through blockade of cyclic GMP‐AMP synthase‐DNA interaction. J Immunol. 2015;194:4089‐4093. [DOI] [PubMed] [Google Scholar]
- 61. Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD. SARS‐CoV‐2 sensitive to type I interferon pretreatment. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maringer K, Fernandez‐sesma A. Cytokine & growth factor reviews message in a bottle: lessons learned from antagonism of STING signalling during RNA virus infection. Cytokine Growth Factor Rev. 2014;25:669‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Austin Taylor M, Bennett M, Kumar V, Schatzle JD. Functional defects of NK cells treated with chloroquine mimic the lytic defects observed in perforin‐deficient mice. J Immunol. 2000;165:5048‐5053. [DOI] [PubMed] [Google Scholar]
- 64. Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheum. 2014;66:319‐326. [DOI] [PubMed] [Google Scholar]
- 65. Yang J, Yang X, Yang J, et al. Hydroxychloroquine inhibits the differentiation of Th17 cells in systemic lupus erythematosus. J Rheumatol. 2018;45(6):818‐826. [DOI] [PubMed] [Google Scholar]
- 66. Oh S, Shin JH, Jang EJ, et al. Anti‐inflammatory activity of chloroquine and amodiaquine through p21‐mediated suppression of T cell proliferation and Th1 cell differentiation. Biochem Biophys Res Commun. 2016;474:345‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155‐166. [DOI] [PubMed] [Google Scholar]
- 68. Mocholi E, Dowling SD, Botbol Y, et al. Autophagy is a tolerance‐avoidance mechanism that modulates TCR‐mediated signaling and cell metabolism to prevent induction of T cell anergy. Cell Rep. 2018;24:1136‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Accapezzato D, Visco V, Francavilla V, et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garulli B, Stillitano MG, Barnaba V, Castrucci MR. Primary CD8+ T‐cell response to soluble ovalbumin is improved by chloroquine treatment in vivo. Clin Vaccine Immunol. 2008;15:1497‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Klempner MS, Styrt B. Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. J Clin Invest. 1983;72:1793‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morrison LA, Braciale VL, Braciale TJ. Distinguishable pathways of viral antigen presentation to T lymphocytes. Immunol Res. 1986;5:294‐304. [DOI] [PubMed] [Google Scholar]
- 73. Borba EF, Saad CGS, Pasoto SG, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology. 2012;51:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 74. Murugan R, Buchauer L, Triller G, et al. Clonal selection drives protective memory B cell responses in controlled human malaria infection. Sci Immunol. 2018;3(20):eaap8029. [DOI] [PubMed] [Google Scholar]
- 75. Ly A, Liao Y, Pietrzak H, et al. Transcription factor T‐bet in B cells modulates germinal center polarization and antibody affinity maturation in response to malaria. Cell Rep. 2019;29:2257‐2269.e6. [DOI] [PubMed] [Google Scholar]
- 76. Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody‐dependent enhancement of coronavirus entry. J Virol. 2020;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bradley‐Moore AM, Greenwood BM, Bradley AK, et al. Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann Trop Med Parasitol. 1985;79:563‐573. [DOI] [PubMed] [Google Scholar]
- 78. Gilles HM, Greenwood BM, Greenwood AM, et al. The Malumfashi project–an epidemiological, clinical and laboratory study. Trans R Soc Trop Med Hyg. 1983;77:24‐31. [DOI] [PubMed] [Google Scholar]
- 79. Paffenbarger RS, Kampert JB, Lee IM, Hyde RT, Leung RW, Wing AL. Changes in physical activity and other lifeway patterns influencing longevity. Med Sci Sports Exerc. 1994;26:857‐865. [PubMed] [Google Scholar]
- 80. Kollaritsch H, Que JU, Kunz C, Wiedermann G, Herzog C, Cryz SJ Jr. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J Infect Dis. 1997;175:871‐875. [DOI] [PubMed] [Google Scholar]
- 81. Eng EO, Chew JSW, Jin PL, et al. In vitro inhibition of human influenza A virus replication by chloroquine. Virol J. 2006;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


