Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causes coronavirus disease 2019 (COVID‐19) that manifests with variable severity. 1 A subset of symptomatic individuals develop proinflammatory or prothrombotic profiles requiring additional testing and interventions.2, 3, 4, 5 It is unclear which COVID‐19 patients will ultimately develop severe disease that would have benefitted from early and aggressive interventions.
Fig 1.
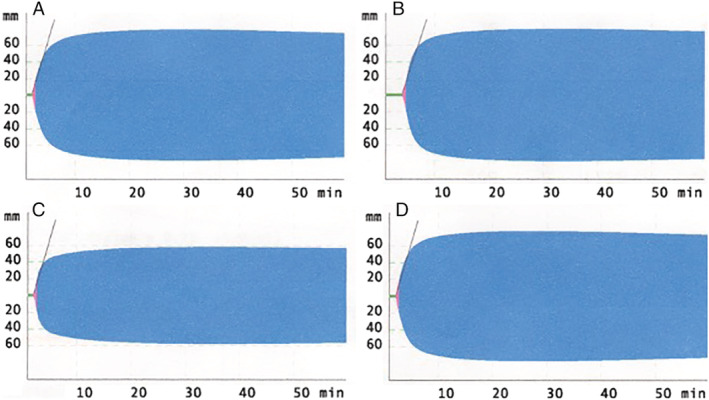
Viscoelastic testing tracings from an intensive care unit patient with severe COVID‐19. (A) EXTEM tracing; (B) INTEM tracing; (C) FIBTEM tracing; (D) APTEM tracing.
A 63‐year‐old man was admitted with rapidly progressive COVID‐19 pneumonia with hypoxia. Given the patient's worsening clinical status, laboratory coagulation analysis, including viscoelastic testing by rotational thromboelastometry (ROTEM delta, Instrumentation Laboratory Co., Bedford, MA), was performed immediately upon hospital admission. He subsequently developed acute respiratory distress syndrome and shock that required mechanical ventilation and vasopressor support.
Routine coagulation testing demonstrated a prothrombin time (PT) of 12.2 seconds (normal 9.4‐15.4 seconds), a partial thromboplastin time (PTT) of 30 seconds (normal 26‐38 seconds), and D‐dimers of 2143 ng/mL fibrinogen equivalent units (FEU; normal <600 ng/mL FEU), the last associated with severe COVID‐19. 6 Viscoelastic testing demonstrated a hypercoagulable profile (see Fig. 1). 7 In particular, there was elevated maximum clot firmness observed on EXTEM (78 mm), INTEM (79 mm), and FIBTEM (58 mm) tracings (normal 52‐70 mm, 51‐72 mm, and 10‐24 mm, respectively), and clot formation time was shortened on the EXTEM tracing (39 seconds, normal 48‐127 seconds) with an increased α angle (82°, normal 65‐80°). The patient was placed on 7500 units of subcutaneous unfractionated heparin every 8 hours for thrombosis prevention based on his critical illness and viscoelastic testing results. The patient still requires mechanical ventilation and vasopressor support; he has not developed any overt thrombotic or bleeding events, and D‐dimers have decreased (1294 ng/mL FEU).
These preliminary findings suggest that viscoelastic testing may have a role in rapidly identifying patients with severe COVID‐19. Other viscoelastic methods of assessing clot firmness could also be used in COVID‐19, such as thromboelastography and resonance sonorheometry.8, 9 Measuring D‐dimer or fibrinogen concentrations could assess this pathologic phenomenon if viscoelastic testing was unavailable.10, 11 The utility of viscoelastic testing in COVID‐19 needs to be further assessed to better understand the usefulness and limitations of this technology in these critically ill patients with a hypercoagulable state. 12
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) pandemic [monograph on the internet]. World Health Organization; 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Obi AT, Barnes GD, Wakefield TW, et al. Practical diagnosis and treatment of suspected venous thromboembolism during COVID‐19 Pandemic. J Vasc Surg Venous Lymphat Disord 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thachil J, Tang N, Satoshi G, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost 2020;18:1023‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID‐19. J Infect 2020. 10.1016/j.jinf.2020.03.037. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Favaloro EJ. D‐dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost 2020;120:876‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akay OM. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by Rotational Thromboelastometry (ROTEM). Clin Appl Thromb Hemost 2018;24:850‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020. 10.1111/jth.14850. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baryshnikova E, Di Dedda U, Ranucci M. A comparative study of SEER sonorheometry versus standard coagulation tests, rotational thromboelastometry, and multiple electrode aggregometry in cardiac surgery. J Cardiothorac Vasc Anesth 2019;33:1590‐8. [DOI] [PubMed] [Google Scholar]
- 10. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med 2020. 10.1515/cclm-2020-0188. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies GR, Lawrence M, Pillai S, et al. The effect of sepsis and septic shock on the viscoelastic properties of clot quality and mass using rotational thromboelastometry: a prospective observational study. J Crit Care 2018;44:7‐11. [DOI] [PubMed] [Google Scholar]


